From Wikipedia, the free encyclopedia
This article is about a specific mixture of the two amphetamine enantiomers. For more information on the amphetamine compound and other mixtures of the enantiomers, see Amphetamine.
 | |
 | |
| Combination of | |
|---|---|
| amphetamine aspartate monohydrate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| amphetamine sulfate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| dextroamphetamine saccharate | 25% – stimulant (0% levo; 25% dextro) |
| dextroamphetamine sulfate | 25% – stimulant (0% levo; 25% dextro) |
| Clinical data | |
| Trade names | Adderall, Adderall XR |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601234 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Physical: none Psychological: moderate |
| Addiction liability | Moderate |
| Routes of administration | Oral, insufflation, rectal, sublingual |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | 300-62-9 |
| ATC code | N06BA02 (WHO) N06BA01 (WHO) |
| PubChem | CID 3007 |
| IUPHAR/BPS | 4804 |
| DrugBank | DB00182 |
| ChemSpider | 13852819 |
| KEGG | D03740 |
| ChEBI | CHEBI:2679 |
| ChEMBL | CHEMBL405 |
| (verify) | |
Adderall[note 1] is a combination drug containing salts of the two enantiomers of amphetamine, a central nervous system(CNS) stimulant of the phenethylamine class. Adderall is used in the treatment of attention deficit hyperactivity disorder(ADHD) and narcolepsy. It is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. By salt content, the active ingredients of Adderall are 25% levoamphetamine salts (the levorotary or 'left-handed' enantiomer) and 75% dextroamphetamine salts (the dextrorotary or 'right-handed' enantiomer).[note 2][sources 1]
Adderall is generally well-tolerated and effective in treating the symptoms of ADHD and narcolepsy. At therapeutic doses, Adderall causes emotional and cognitive effects such as euphoria, change in desire for sex, increased wakefulness, and improved cognitive control. At these doses, it induces physical effects such as decreased reaction time, fatigue resistance, and increased muscle strength. In contrast, much larger doses of Adderall can impair cognitive control, cause rapid muscle breakdown, or induce a psychosis (e.g., delusions and paranoia). The side effects of Adderall vary widely among individuals, but most commonly include insomnia, dry mouth, and loss of appetite. The risk of developing an addiction is insignificant when Adderall is used as prescribed at fairly low daily doses, such as those used for treating ADHD; however, the routine use of Adderall in larger daily doses poses a significant risk of addiction due to the pronounced reinforcing effects that are present at higher doses. Recreational doses of Adderall are generally much larger than prescribed therapeutic doses, and carry a far greater risk of serious adverse effects.[sources 2]
The two amphetamine enantiomers that compose Adderall (i.e., levoamphetamine and dextroamphetamine) alleviate the symptoms of ADHD and narcolepsy by increasing the activity of the neurotransmitters norepinephrine and dopamine in the brain, which results from their interactions with trace amine associated receptor 1 (TAAR1) and vesicular monoamine transporter 2 (VMAT2) in neurons. Dextroamphetamine is a more potent CNS stimulant than levoamphetamine, but levoamphetamine has slightly stronger cardiovascular and peripheral effects and a longer elimination half-life (i.e., it remains in the body longer) than dextroamphetamine. The levoamphetamine component of Adderall has been reported to improve the treatment response in some individuals relative to dextroamphetamine alone. Adderall's active ingredient, amphetamine, shares many chemical and pharmacological properties with the human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter of which is a positional isomer of amphetamine.[sources 3]
Uses[edit]
Medical[edit]
Adderall is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder).[2][6] Long-term amphetamine exposure at sufficiently high doses in some animal species is known to produce abnormal dopamine system development or nerve damage,[24][25] but, in humans with ADHD, pharmaceutical amphetamines appear to improve brain development and nerve growth.[26][27][28] Reviews of magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleusof the basal ganglia.[26][27][28]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term amphetamine use for ADHD.[29][30][31] Controlled trials spanning two years have demonstrated treatment effectiveness and safety.[29][31] One review highlighted a nine-month randomized controlled trial in children with ADHD that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity.[29]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems;[16] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the locus coeruleus and prefrontal cortex.[16]Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[7][16][32] Approximately 80% of those who use these stimulants see improvements in ADHD symptoms.[33] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[34][35] The Cochrane Collaboration's reviews[note 3] on the treatment of ADHD in children, adolescents, and adults with pharmaceutical amphetamines stated that while these drugs improve short-term symptoms, they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[37][38] A Cochrane Collaboration review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[39]
Adderall is available as immediate release tablets or extended-release capsules.[6][40] The extended release capsule is generally used in the morning.[41] The extended release formulation available under the brand Adderall XR is designed to provide a therapeutic effect and plasma concentrations identical to taking two doses 4 hours apart.[40]
Enhancing performance[edit]
In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, amphetamine produces modest, unambiguous improvements in cognition, including working memory, episodic memory, inhibitory control and some aspects of attention, in normal healthy adults;[42][43] the cognition-enhancing effects of amphetamine are known to occur through its indirect activation of both dopamine receptor D1 and adrenoceptor α2 in the prefrontal cortex.[7][42] A systematic review from 2014 noted that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information.[44]Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals.[7][45]Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[7][46][47] Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid.[7][47][48] Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for performance enhancement rather than as recreational drugs.[49][50][51] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.[7][47]
Amphetamine is used by some athletes for its psychological and athletic performance-enhancing effects, such as increased endurance and alertness;[8][20] however, non-medical amphetamine use is prohibited at sporting events that are regulated by collegiate, national, and international anti-doping agencies.[52][53] In healthy people at oral therapeutic doses, amphetamine has been shown to increase muscle strength, acceleration, athletic performance in anaerobic conditions, and endurance (i.e., it delays the onset of fatigue), while improving reaction time.[8][54][55] Amphetamine improves endurance and reaction time primarily through reuptake inhibition and effluxion of dopamine in the central nervous system.[54][55][56] Amphetamine and other dopaminergic drugs also increase power output at fixed levels of perceived exertion by overriding a "safety switch" that allows the core temperature limit to increase in order to access a reserve capacity that is normally off-limits.[55][57][58] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[8][54] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[9][10][54]
Adderall has been banned in the National Football League (NFL), Major League Baseball (MLB), National Basketball Association (NBA), and the National Collegiate Athletics Association (NCAA).[59] In leagues such as the NFL, there is a very rigorous process required to obtain an exemption to this rule even when the athlete has been medically prescribed the drug by their physician.[59]
Recreational[edit]
Adderall is considered to have a high potential for misuse as a recreational drug.[60][61] Adderall tablets can be crushed and snorted, or dissolved in water and injected.[62]Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[62]
Contraindications[edit]
According to the International Programme on Chemical Safety (IPCS) and United States Food and Drug Administration (USFDA),[note 4] amphetamine is contraindicated in people with a history of drug abuse,[note 5] heart disease, severe agitation, or severe anxiety.[64][65] It is also contraindicated in people currently experiencing arteriosclerosis (hardening of the arteries), glaucoma (increased eye pressure), hyperthyroidism (excessive production of thyroid hormone), or moderate to severe hypertension.[64][65][66] People who have experienced allergic reactions to other stimulants in the past or who are taking monoamine oxidase inhibitors (MAOIs) are advised not to take amphetamine,[64][65] although safe concurrent use of amphetamine and monoamine oxidase inhibitors has been documented.[67][68] These agencies also state that anyone with anorexia nervosa, bipolar disorder, depression, hypertension, liver or kidney problems, mania, psychosis, Raynaud's phenomenon, seizures, thyroid problems, tics, or Tourette syndrome should monitor their symptoms while taking amphetamine.[64][65] Evidence from human studies indicates that therapeutic amphetamine use does not cause developmental abnormalities in the fetus or newborns (i.e., it is not a human teratogen), but amphetamine abuse does pose risks to the fetus.[65] Amphetamine has also been shown to pass into breast milk, so the IPCS and USFDA advise mothers to avoid breastfeeding when using it.[64][65] Due to the potential for reversible growth impairments,[note 6] the USFDA advises monitoring the height and weight of children and adolescents prescribed an amphetamine pharmaceutical.[64]
Side effects[edit]
The side effects of Adderall are many and varied, but the amount of substance consumed is the primary factor in determining the likelihood and severity of side effects.[9][10][20] Adderall is currently approved for long-term therapeutic use by the USFDA.[10] Recreational use of Adderall generally involves far larger doses and is therefore significantly more dangerous, involving a much greater risk of serious side effects.[20]
Physical
At normal therapeutic doses, the physical side effects of amphetamine vary widely by age and from person to person.[10] Cardiovascular side effects can include hypertension or hypotension from a vasovagal response, Raynaud's phenomenon (reduced blood flow to extremities), and tachycardia (increased heart rate).[10][20][69]Sexual side effects in males may include erectile dysfunction, frequent erections, or prolonged erections.[10] Abdominal side effects may include abdominal pain, appetite loss, nausea, and weight loss.[10][70] Other potential side effects include blurred vision, dry mouth, excessive grinding of the teeth, nosebleed, profuse sweating, rhinitis medicamentosa (drug-induced nasal congestion), reduced seizure threshold, and tics (a type of movement disorder).[sources 4] Dangerous physical side effects are rare at typical pharmaceutical doses.[20]
Amphetamine stimulates the medullary respiratory centers, producing faster and deeper breaths.[20] In a normal person at therapeutic doses, this effect is usually not noticeable, but when respiration is already compromised, it may be evident.[20] Amphetamine also induces contraction in the urinary bladder sphincter, the muscle which controls urination, which can result in difficulty urinating.[20] This effect can be useful in treating bed wetting and loss of bladder control.[20] The effects of amphetamine on the gastrointestinal tract are unpredictable.[20] If intestinal activity is high, amphetamine may reduce gastrointestinal motility (the rate at which content moves through the digestive system);[20] however, amphetamine may increase motility when the smooth muscle of the tract is relaxed.[20] Amphetamine also has a slight analgesic effect and can enhance the pain relieving effects of opioids.[20]
USFDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of amphetamine or other ADHD stimulants.[sources 5]
Psychological
Common psychological effects of therapeutic doses can include increased alertness, apprehension, concentration, decreased sense of fatigue, mood swings (elated mood followed by mildly depressed mood), increased initiative, insomnia or wakefulness, self-confidence, and sociability.[10][20] Less common side effects include anxiety, change in libido, grandiosity, irritability, repetitive or obsessive behaviors, and restlessness;[sources 6] these effects depend on the user's personality and current mental state.[20] Amphetamine psychosis (e.g., delusions and paranoia) can occur in heavy users.[9][10][11] Although very rare, this psychosis can also occur at therapeutic doses during long-term therapy.[9][10][12] According to the USFDA, "there is no systematic evidence" that stimulants produce aggressive behavior or hostility.[10]
Amphetamine has also been shown to produce a conditioned place preference in humans taking therapeutic doses,[37][77] meaning that individuals acquire a preference for spending time in places where they have previously used amphetamine.[77][78]
Overdose[edit]
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[65][79] The severity of overdose symptoms increases with dosage and decreases with drug tolerance to amphetamine.[20][65] Tolerant individuals have been known to take as much as 5 grams of amphetamine in a day, which is roughly 100 times the maximum daily therapeutic dose.[65] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[9][20] In 2013, overdose on amphetamine, methamphetamine, and other compounds implicated in an "amphetamine use disorder" resulted in an estimated 3,788 deaths worldwide (3,425–4,145 deaths, 95% confidence).[note 7][80]
Pathological overactivation of the mesolimbic pathway, a dopamine pathway that connects the ventral tegmental area to the nucleus accumbens, plays a central role in amphetamine addiction.[81][82] Individuals who frequently overdose on amphetamine during recreational use have a high risk of developing an amphetamine addiction, since repeated overdoses gradually increase the level of accumbal ΔFosB, a "molecular switch" and "master control protein" for addiction.[83][84][85] Once nucleus accumbens ΔFosB is sufficiently overexpressed, it begins to increase the severity of addictive behavior (i.e., compulsive drug-seeking) with further increases in its expression.[83][86] While there are currently no effective drugs for treating amphetamine addiction, regularly engaging in sustained aerobic exercise appears to reduce the risk of developing such an addiction.[87][88] Sustained aerobic exercise on a regular basis also appears to be an effective treatment for amphetamine addiction;[86][87][89]exercise therapy improves clinical treatment outcomes and may be used as a combination therapy with cognitive behavioral therapy, which is currently the best clinical treatment available.[87][89][90]
| System | Minor or moderate overdose[9][20][65] | Severe overdose[sources 7] |
|---|---|---|
| Cardiovascular |
| |
| Central nervous system |
|
|
| Musculoskeletal |
| |
| Respiratory |
|
|
| Urinary |
| |
| Other |
|
|
Addiction
| Addiction and dependence glossary[78][84][93][94] |
|---|
| • addiction – a medical condition characterized by compulsive engagement in rewarding stimuli despite adverse consequences |
| • addictive behavior – a behavior that is both rewarding and reinforcing |
| • addictive drug – a drug that is both rewarding and reinforcing |
| • dependence – an adaptive state associated with a withdrawal syndrome upon cessation of repeated exposure to a stimulus (e.g., drug intake) |
| • drug sensitization or reverse tolerance – the escalating effect of a drug resulting from repeated administration at a given dose |
| • drug withdrawal – symptoms that occur upon cessation of repeated drug use |
| • physical dependence – dependence that involves persistent physical–somatic withdrawal symptoms (e.g., fatigue and delirium tremens) |
| • psychological dependence – dependence that involves emotional–motivational withdrawal symptoms (e.g., dysphoria and anhedonia) |
| • reinforcing stimuli – stimuli that increase the probability of repeating behaviors paired with them |
| • rewarding stimuli – stimuli that the brain interprets as intrinsically positive or as something to be approached |
| • sensitization – an amplified response to a stimulus resulting from repeated exposure to it |
| • substance use disorder - a condition in which the use of substances leads to clinically and functionally significant impairment or distress |
| • tolerance – the diminishing effect of a drug resulting from repeated administration at a given dose |
| (edit | history) |
Addiction is a serious risk with heavy recreational amphetamine use but is unlikely to arise from typical long-term medical use at therapeutic doses.[13][14][15] Drug tolerance develops rapidly in amphetamine abuse (i.e., a recreational amphetamine overdose), so periods of extended use require increasingly larger doses of the drug in order to achieve the same effect.[95][96]
Biomolecular mechanisms
Current models of addiction from chronic drug use involve alterations in gene expression in certain parts of the brain, particularly the nucleus accumbens.[97][98][99] The most important transcription factors[note 8]that produce these alterations are ΔFosB, cAMP response element binding protein (CREB), and nuclear factor kappa B (NF-κB).[98] ΔFosB plays a crucial role in the development of drug addictions, since its overexpression in D1-type medium spiny neurons in the nucleus accumbens is necessary and sufficient[note 9] for most of the behavioral and neural adaptations that arise from addiction.[83][84][98]Once ΔFosB is sufficiently overexpressed, it induces an addictive state that becomes increasingly more severe with further increases in ΔFosB expression.[83][84] It has been implicated in addictions to alcohol, cannabinoids, cocaine, methylphenidate, nicotine, opioids, phencyclidine, propofol, and substituted amphetamines, among others.[sources 8]
ΔJunD, a transcription factor, and G9a, a histone methyltransferase enzyme, both directly oppose the induction of ΔFosB in the nucleus accumbens (i.e., they oppose increases in its expression).[84][98][103]Sufficiently overexpressing ΔJunD in the nucleus accumbens with viral vectors can completely block many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[98] ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[86][98][104] Since both natural rewards and addictive drugs induce expression of ΔFosB (i.e., they cause the brain to produce more of it), chronic acquisition of these rewards can result in a similar pathological state of addiction.[86][98] Consequently, ΔFosB is the most significant factor involved in both amphetamine addiction and amphetamine-induced sex addictions, which are compulsive sexual behaviors that result from excessive sexual activity and amphetamine use.[86][105][106] These sex addictions are associated with a dopamine dysregulation syndrome which occurs in some patients taking dopaminergic drugs.[86][104]
The effects of amphetamine on gene regulation are both dose- and route-dependent.[99] Most of the research on gene regulation and addiction is based upon animal studies with intravenous amphetamine administration at very high doses.[99] The few studies that have used equivalent (weight-adjusted) human therapeutic doses and oral administration show that these changes, if they occur, are relatively minor.[99] This suggests that medical use of amphetamine does not significantly affect gene regulation.[99]
Pharmacological treatments
Further information: Addiction § Research
As of May 2014, there is no effective pharmacotherapy for amphetamine addiction.[107][108][109] Reviews from 2015 and 2016 indicated that TAAR1-selective agonists have significant therapeutic potential as a treatment for psychostimulant addictions;[110][111] however, as of February 2016, the only compounds which are known to function as TAAR1-selective agonists are experimental drugs.[110][111] Amphetamine addiction is largely mediated through increased activation of dopamine receptors and co-localized NMDA receptors[note 10] in the nucleus accumbens;[82] magnesium ions inhibit NMDA receptors by blocking the receptor calcium channel.[82][112] One review suggested that, based upon animal testing, pathological (addiction-inducing) psychostimulant use significantly reduces the level of intracellular magnesium throughout the brain.[82] Supplemental magnesium[note 11] treatment has been shown to reduce amphetamine self-administration (i.e., doses given to oneself) in humans, but it is not an effective monotherapy for amphetamine addiction.[82]
Behavioral treatments
Cognitive behavioral therapy is currently the most effective clinical treatment for psychostimulant addictions.[90] Additionally, research on the neurobiological effects of physical exercise suggests that daily aerobic exercise, especially endurance exercise (e.g., marathon running), prevents the development of drug addiction and is an effective adjunct therapy (i.e., a supplemental treatment) for amphetamine addiction.[87][88][89] Exercise leads to better treatment outcomes when used as an adjunct treatment, particularly for psychostimulant addictions.[87][89] In particular, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces increased dopamine receptor D2 (DRD2) density in the striatum.[86] This is the opposite of pathological stimulant use, which induces decreased striatal DRD2 density.[86] One review noted that exercise may also prevent the development of a drug addiction by altering ΔFosB or c-Fos immunoreactivity in the striatum or other parts of the reward system.[88]
Dependence and withdrawal
According to another Cochrane Collaboration review on withdrawal in individuals who compulsively use amphetamine and methamphetamine, "when chronic heavy users abruptly discontinue amphetamine use, many report a time-limited withdrawal syndrome that occurs within 24 hours of their last dose."[113] This review noted that withdrawal symptoms in chronic, high-dose users are frequent, occurring in up to 87.6% of cases, and persist for three to four weeks with a marked "crash" phase occurring during the first week.[113] Amphetamine withdrawal symptoms can include anxiety, drug craving, depressed mood, fatigue, increased appetite, increased movement or decreased movement, lack of motivation, sleeplessness or sleepiness, and lucid dreams.[113] The review indicated that withdrawal symptoms are associated with the degree of dependence, suggesting that therapeutic use would result in far milder discontinuation symptoms.[113] Manufacturer prescribing information does not indicate the presence of withdrawal symptoms following discontinuation of amphetamine use after an extended period at therapeutic doses.[66][114][115]
Toxicity and psychosis
See also: Stimulant psychosis
In rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized by reduced transporter and receptor function.[116] There is no evidence that amphetamine is directly neurotoxic in humans.[117][118] However, large doses of amphetamine may cause indirect neurotoxicity as a result of increased oxidative stress from reactive oxygen species and autoxidation of dopamine.[24][119][120]
A severe amphetamine overdose can result in a stimulant psychosis that may involve a variety of symptoms, such as paranoia and delusions.[11] A Cochrane Collaboration review on treatment for amphetamine, dextroamphetamine, and methamphetamine psychosis states that about 5–15% of users fail to recover completely.[11][121] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[11] Psychosis very rarely arises from therapeutic use.[12][64]
Interactions[edit]
- Monoamine oxidase inhibitors (MAOIs) taken with Adderall may result in a hypertensive crisis if taken within two weeks after last use of an MAOI type drug.[122]
- Inhibitors of enzymes that directly metabolize amphetamine (particularly FMO3 and CYP2D6) will prolong the elimination of amphetamine.[122][123]
- Stimulants and antidepressants (sedatives and depressants) may increase (decrease) the drug effects of Adderall, and vice versa.[122]
- Dietary pH affects the absorption and elimination half-life of Adderall; an alkaline diet increases the rate of absorption and decreases the rate of excretion, while acidic diets decrease absorption and increase excretion rates.[122]
- Proton-pump inhibitors (PPIs) modify the pharmacokinetics of Adderall XR. Co-administration requires monitoring for changes in clinical effect.[122]
- Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 12][127]
Pharmacology[edit]
Mechanism of action[edit]
For a more complete and detailed description of amphetamine pharmacodynamics, see Amphetamine § Pharmacodynamics.
Amphetamine, the active ingredient of Adderall, works primarily by increasing the activity of the neurotransmitters dopamine and norepinephrine in the brain.[16][32] It also triggers the release of several other hormones (e.g., epinephrine) and neurotransmitters (e.g., serotonin and histamine) as well as the synthesis of certain neuropeptides (e.g., cocaine and amphetamine regulated transcript [CART] peptides),.[18][128] Both active ingredients of Adderall, dextroamphetamine and levoamphetamine, bind to the same biological targets,[20][21] but their binding affinities (that is, potency) differ somewhat.[20][21] Dextroamphetamine and levoamphetamine are both potent full agonists (activating compounds) of trace amine-associated receptor 1(TAAR1) and interact with vesicular monoamine transporter 2 (VMAT2), with dextroamphetamine being the more potent agonist of TAAR1.[21]Consequently, dextroamphetamine produces more CNS stimulation than levoamphetamine;[21][129] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[20] Levoamphetamine provides Adderall with a quicker onset and longer-lasting effects than dextroamphetamine alone.[130][unreliable medical source?][non-primary source needed] It has been reported that certain children have a better clinical response to levoamphetamine.[22][23]
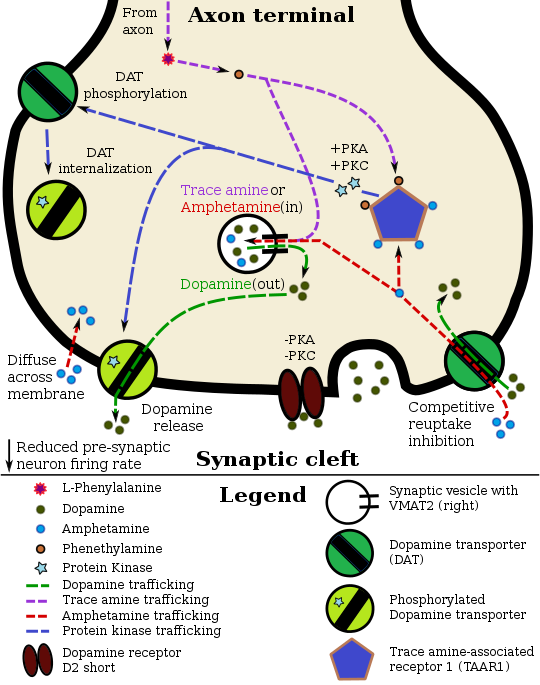
In the absence of amphetamine, VMAT2 will normally move monoamines(e.g., dopamine, histamine, serotonin, norepinephrine, etc.) from the intracellular fluid of a monoamine neuron into its synaptic vesicles, which are essentially chemical storage units inside a neuron.[18] When amphetamine enters a neuron and interacts with VMAT2, the transporter reverses its direction of transport, thereby releasing stored monoamines inside synaptic vesicles back into the neuron's intracellular fluid.[18] Meanwhile, when amphetamine activates TAAR1, the receptor causes the neuron's cell membrane-bound monoamine transporters (i.e., the dopamine transporter, norepinephrine transporter, or serotonin transporter) to either stop transporting molecules altogether (via internalization) or even transport them in reverse;[17] in other words, the reversed membrane transporter will push dopamine, norepinephrine, and serotonin out of the neuron's intracellular fluid and into the synaptic cleft.[17] In summary, by interacting with both VMAT2 and TAAR1, amphetamine releases neurotransmitters from synaptic vesicles (the effect from VMAT2) into the intracellular fluid where they subsequently exit the neuron through the membrane-bound, reversed monoamine transporters (the effect from TAAR1).[17][18]
Pharmacokinetics[edit]
The oral bioavailability of amphetamine varies with gastrointestinal pH;[122] it is well absorbed from the gut, and bioavailability is typically over 75% for dextroamphetamine.[131] Amphetamine is a weak base with a pKa of 9.9;[5] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[5][122] Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic(salt) form, and less is absorbed.[5] Approximately 15–40% of amphetamine circulating in the bloodstream is bound to plasma proteins.[132]
The half-life of amphetamine enantiomers differ and vary with urine pH.[5] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[5] An acidic diet will reduce the enantiomer half-lives to 8–11 hours; an alkaline diet will increase the range to 16–31 hours.[133][134] The biological half-life is longer and distribution volumes are larger in amphetamine dependent individuals.[134] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[5] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[5] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[5] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[5]Amphetamine is usually eliminated within two days of the last oral dose.[133]
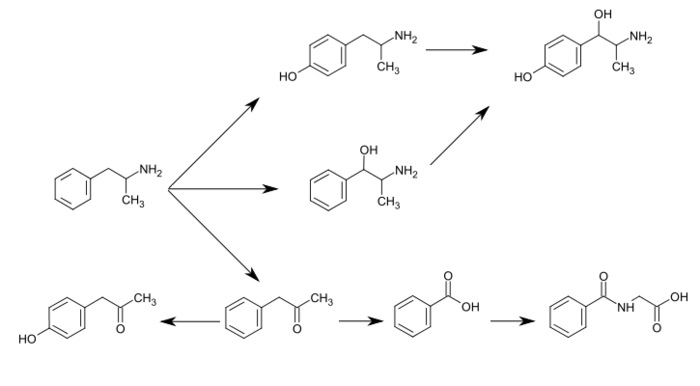
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 9] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[5][133][139] Among these metabolites, the active sympathomimetics are 4‑hydroxyamphetamine,[142] 4‑hydroxynorephedrine,[143] and norephedrine.[144] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[5][133] The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
Related endogenous compounds[edit]
For more details on related compounds, see Trace amine.
Amphetamine has a very similar structure and function to the endogenous trace amines, which are naturally occurring neurotransmitter molecules produced in the human body and brain.[17][19] Among this group, the most closely related compounds are phenethylamine, the parent compound of amphetamine, and N-methylphenethylamine, an isomer of amphetamine (i.e., it has an identical molecular formula).[17][19][145] In humans, phenethylamine is produced directly from L-phenylalanine by the aromatic amino acid decarboxylase (AADC) enzyme, which converts L-DOPA into dopamine as well.[19][145] In turn, N‑methylphenethylamine is metabolized from phenethylamine by phenylethanolamine N-methyltransferase, the same enzyme that metabolizes norepinephrine into epinephrine.[19][145] Like amphetamine, both phenethylamine and N‑methylphenethylamine regulate monoamine neurotransmission via TAAR1;[17][145] unlike amphetamine, both of these substances are broken down by monoamine oxidase B, and therefore have a shorter half-life than amphetamine.[19][145]
History, society, and culture[edit]
Main article: History and culture of substituted amphetamines
Richwood Pharmaceuticals, which later merged with Shire plc, introduced the current Adderall brand in 1996 as an instant-release tablet.[146] In 2006, Shire agreed to sell rights to the Adderall name for this instant-release medication to Duramed Pharmaceuticals.[147] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 during their acquisition of Barr Pharmaceuticals, including Barr's Duramed division.[148]
The first generic version of Adderall IR was introduced to market in 2002.[1] Later on, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the drug beginning in April 2009.[1][149]
Commercial formulation[edit]
Chemically, Adderall is a mixture of several amphetamine salts; specifically, it is composed of equal parts (by mass) of amphetamine aspartate monohydrate, amphetamine sulfate, dextroamphetamine sulfate, and dextroamphetamine saccharate.[40] This drug mixture has slightly stronger CNS effects than racemic amphetamine due to the higher proportion of dextroamphetamine.[17][20] Adderall is produced as both an immediate release (IR) and extended release (XR) formulation.[1][6][40] As of December 2013, ten different companies have produced generic Adderall IR at one point, while Teva Pharmaceutical Industries, Actavis, and Barr Pharmaceuticalscurrently manufacture generic Adderall XR.[1] Shire plc, the company that held the original patent for Adderall and Adderall XR, still manufactures brand name Adderall XR, but not Adderall IR.[1]
Comparison to other formulations[edit]
Adderall is one of several formulations of pharmaceutical amphetamine, including singular or mixed enantiomers and as an enantiomer prodrug. The table below compares these medications (based on US approved forms):
| drug | formula | molecular mass [note 13] | amphetamine base [note 14] | amphetamine base in equal doses | doses with equal base content [note 15] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[151][152] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[153] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[151][152] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[153] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[154] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[155] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[156] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[note 16][70] | C9H13N | |||||||||
Past formulations[edit]
Rexar, a pharmaceutical company, reformulated another drug, branded as Obetrol, and continued to sell this new formulation under the same brand name. This new unapproved formulation was later rebranded and sold as Adderall by Richwood after it acquired Rexar resulting in FDA warning in 1994. Richwood submitted this formulation as NDA 11-522 and Adderall gained FDA approval for the treatment of attention-deficit/hyperactivity disorder on 13 February 1996.[157]
Legal status[edit]
- In Canada, amphetamines are in Schedule I of the Controlled Drugs and Substances Act, and can only be obtained by prescription.[158]
- In Japan, the use, production, and import of any medicine containing amphetamine are prohibited.[159]
- In South Korea, amphetamines are prohibited.[160]
- In Thailand, Amphetamines are classified as Type 1 Narcotics.[161]
- In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in prison and an unlimited fine.[162]
- In the United States, amphetamine is a Schedule II prescription drug, classified as a CNS stimulant.[163]
- Internationally (United Nations), amphetamine is in Schedule II of the Convention on Psychotropic Substances.[164][165]


It is no secret that I have a very deep and personal relationship with God. I have pushed and resisted that relationship this past year through all the bullshit I have had to go through living with Herpes but once again, God is bigger than my stubbornness and broke through that outbreak cold sore and all I had Genital Herpes. For me personally, hearing over and over how I am not good enough has really invaded my mind in the worst way possible. I completely shut down and I was just waking up like is this how life going to end this temporary herpes outbreak “fuck everybody with herpes if you know what I mean” but let's be honest here...
ОтветитьУдалитьIt is a cowardly to say no to herbal medicine. It is fear based. And it is dishonest to what my heart wants. Don't build a wall around yourself because you are afraid of herbals made or taking a bold step especially when it's come to health issues and getting cure. So many young men/ women tell me over and over that Dr Itua is going to scam me but I give him a try to today I feel like no one will ever convince me about herbal medicine I accept Dr Itua herbal medicine because it's cure my herpes just two weeks of drinking it and i have been living for a year and months now I experience outbreak no more, You can contact him if you need his herbal medicine for any such diseases like, Herpes, Schizophrenia,Cancer,Scoliosis,Fibromyalgia,Fluoroquinolone Toxicity Syndrome Fibrodysplasia Ossificans Progressiva.Fatal Familial Insomnia Factor V Leiden Mutation ,Epilepsy Dupuytren's disease,Desmoplastic,Diabetes ,Coeliac disease,Creutzfeldt–Jakob,,Lyme Disease,Epilepsy, ,ALS,Hepatitis,Copd,Parkinson disease.Genetic disease,Fibrodysplasia disease,Fibrodysplasia Ossificans Men/Woman infertility, bowel disease ,Huntington's disease ,Diabetes,Fibroid. disease,Lupus,Lipoid Storage diseases( Gauchers disease),Polycystic Disease.,Cerebral Amyloid Angiopathy, Ataxia,Cirrhosis of Liver,Arthritis,Amyotrophic Lateral Sclerosis,Alzheimer's disease,Adrenocortical carcinoma.Asthma,Allergic,HIV, Epilepsy, Infertility, Love Spell,. Email..drituaherbalcenter@gmail.com then what's app.+2348149277967.... My advice to any sick men/women out there is simple... Be Always an open book. Be gut wrenching honest about yourself, your situation, and what you are all about. Don't hold anything back. Holding back will get you nowhere...maybe a one way ticket to lonelyville and that is NOT somewhere you want to be. So my final truth...and I'm just starting to grasp this one..