What will be included within this article (for those that do not care about how I arrived at my recommended stack design, and just want the answer, feel free to skip to “practical application section)
- History of GH and Fat Loss
- What is Lipolysis?
- The Stress Hormone / Relationship of GH and Fasting
- Mechanisms of Action – GH-Mediated-Lipolysis
- Maximum Rate of Lipolysis
- Pharmacokinetics/Pharmacodynamics
- Compound Synergies
- Practical Application and Sample Stack Design
History of GH and Fat Loss
Growth Hormone (GH) is a very potent fat mobilizing agent which has been known to researchers as far back as the 1920s, when primitive animal trials demonstrated that pituitary-treated animals were consistently leaner than untreated control animals [1–2]. However it wasn’t until 1945, when GH was first extracted from the pituitary [3], that scientists truly began to isolate the fat mobilization effects of the pituitary to this specific polypeptide.
What is Lipolysis?
Lipolysis is the physiological process which provides the body with an energy substrate in the form of fatty acids via catabolism of stored triacylglycerol. Triacylglycerol stored within the lipid droplets of adipocytes are hydrolyzed into fatty acids and glycerol, and subsequently released into circulation for oxidation and ATP production [4–5]. It is going to be somewhat important to understand the differences between mobilization and oxidation; however a comprehensive review of lipolysis is beyond the scope of this article.
The Stress Hormone / Relationship of GH and Fasting
GH is a stress hormone, by its very nature, with endogenous secretion magnified during stressful events such as fasting and exercises [6]. For the remainder of this article though, we are going to focus more on the role GH plays during fasting, and how it is applicable to those looking to maximize the lipolytic potential of their exogenous GH stack designs.
The fasted (postabsorptive) period begins roughly six hours after food intake and the body’s primary objectives during this stage are to supply, convert, and conserve fuel substrates for the body. During this period, there is a significant elevation in the rate of endogenous GH secretion which can last for 48-72 hours [7–8]. The elevation of secreted GH is directly related to pulse amplitude whereas frequency of the pulses and inter-pulse trough levels remain essentially unchanged [9]. During fasting, GH is the only anabolic hormone to increase while levels of catabolic hormones (e.g. glucagon, cortisol, epinephrine, etc) all increase.
Mechanisms of Action – GH-Mediated Lipolysis
The elevated rates of GH secretion bring with them numerous metabolic shifts, which are important to understand. The first priority of the body during fasting is to maintain glucose homeostasis, as to provide sufficient glucose to the brain and other tissues (e.g. red blood cells) that are dependent upon this fuel [10]. To achieve this goal, the body shifts its fuel intake preference to fat substrates so that it can simultaneously conserve valuable glucose and amino stores. In parallel to this shift in fuel preference to fat substrates by muscle tissues and the liver, mobilization of glycogen will occur as no dietary intake of glucose is detected. Large amounts of glucose are also released from the liver into the blood to help maintain blood glucose levels in the absence of dietary glucose. This is achievable, in large part, to the simultaneous drop in serum insulin levels which prevents the released glucose from entering muscle and adipose tissues.
Further to this, the elevated GH brings with it a state of insulin resistance, vital to the preservation of valuable glucose reserves. These insulin antagonistic effects that GH brings with it reduce glucose oxidation and conversely the need for gluconeogenic precursors from muscle protein stores [11], effectively killing two birds with one stone. There are a few thoughts on whether GH itself or the elevation in FFAs is primarily responsible for this increased insulin resistance however that begins to head into cellular transport and signaling pathway territory so we will save that discussion for an article on another day. So, to tie up this up, during fasting GH increased secretion helps to mobilize FFAs from adipocytes, down-regulates GLUT-1 to inhibit glucose uptake in peripheral tissues, prevents glucose oxidation via increased insulin resistance, and preserve amino stores both via direct and indirect means.
It is well known that GH influences lipolysis, however the exact mechanisms by which it does remain somewhat elusive. It has been speculated that this may be multi-faceted with GH demonstrating ability to reduce adipose tissue lipoprotein lipase (LPL), stimulate hormone-sensitive lipase (HSL), and antagonize the antilipolytic effects of insulin. Increased HSL expression in adipocytes increases lipolytic potential as HSL is intimately involved in the triacylglycerol hydrolyzation process. Once activated, HSL is translocated to the periphery of the intracellular fat droplet where it hydrolyzes triacylglycerol to FFA and glycerol. It is also recognized by many as the rate-determining enzyme for lipolysis [12]. Worth noting though is that not all studies have universally shown GH to increase HSL mRNA levels in adipocytes however [13–14].
As mentioned, GH has also been shown to have a direct impact on suppressing LPL activity in human adipose tissues, although this has not been demonstrated in skeletal muscle tissues [15–17]. Why this is potentially relevant to someone interested in fat loss is that LPL is directly involved in clearing fatty acids from the bloodstream and subsequently storing them in adipocytes and/or providing them to skeletal muscle tissues for fuel. So, if LPL can be suppressed in adipose tissues, one could speculate less fat substrates will be actively stored (re-esterified) and more will be available for fueling metabolic processes.
Studies done on cultured human adipocytes have demonstrated that GH actually has no direct lipolytic effects [18], but it does significantly increase catecholamine sensitivity in these cells suggesting GH is activating lipolysis at a stage after the involvement of the beta-adrenoceptors and/or the G-proteins. In fact, it is within reason to speculate that GH may be increasing the density of beta-adrenoceptors, which is where things could potentially get very interesting. It has been shown previously that there are “spare” beta-adrenoceptors on human adipocytes and an acute increase in the number of coupled receptors would increase sensitivity and ultimately lipolytic potential [19]. And, in animal models, GH has been shown to specifically increase the expression of β3-adrenergic receptors in adipocytes, followed by the activation of HSL [20]. So, in addition to the directly mediated effects GH possesses, it stands to reason that the use of a beta-adrenergic agonist could very likely create an additive effect upon the lipolytic process.
Maximum Rate of Lipolysis
So as we work to create a stack that maximizes lipolytic potential, is there an actual (or theoretical) limit to the rate at which it will occur? We actually do have an answer; at least as it relates solely to the maximum rate in which intravenously-administered GH can elicit lipolysis [21]. This was found to be a dose of around 3mcgs/kg (corresponding to an average peak GH concentration of 32.4mcgs/liter). The dose was not age or sex-dependent and works out to roughly the equivalent of 1.2-1.5IUs for a 100kg lean male. Dosing anything higher than this does not actually elicit a greater impact upon lipolysis. It just so happens that this is also essentially the upper limit of naturally occurring, endogenous secretory bursts [22]. It is speculated this could be a limitation, or bottleneck, caused at least in part by extra-renal clearance rates in conjunction with circulating GHBP levels [23].
There is some evidence that this is a GH-specific bottleneck, and that combined treatments with catecholamine variants produces an additive effect on lipolysis, greater than either treatment alone [24-27] which further supports the view that GH is a lipolytic-mediator of sorts. Some have referred to this as being a permissive effect on catecholamine-induced lipolysis [28]. Anecdotally, it does seem as if GH plus the presence of catecholamine-stimulating compounds [29] do possess an additive effect upon one another. And we will dive a bit deeper on this later in the article.
Pharmacokinetics / Pharmacodynamics
Pharmacokinetics is how an administered substance moves through the body (e.g. absorption, distribution, and excretion) and pharmacodynamics is the effects an administered substance has on the body [30]. It is quite important to understand both, as it relates to GH, in order to fully maximize its lipolytic potential and ensure the administered GH is optimized and prevent protocol designs which aren’t properly configured. GH can often be quite expensive to acquire, so it would be in our best interests to try not to waste it.
Routes of administration change the pharmacokinetics of GH greatly. As discussed earlier, endogenous GH is secreted in a pulsatile manner with its presence in serum quickly eliminated via the body’s natural negative regulating feedbacks. In order to most closely mimic this secretory behavior, one would need to use intravenous administration once every 2-3 hours which is the length of time it takes for the ultra-short feedback (GHRH inhibiting its own secretion) to clear. However, unless under doctor supervision, this is not something I either encourage or support and so this article will instead focus on the two most popularly used GH administration methods; subcutaneous and intramuscular.
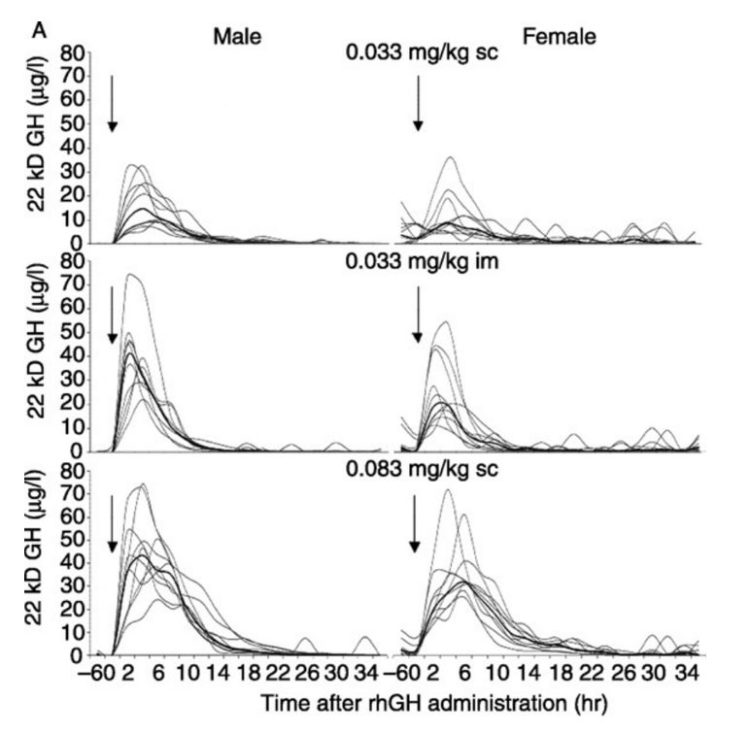
I’m actually going to attach a chart at the end of this article that comes from a very well-designed study [31] comparing the pharmacodynamics and pharmacokinetic properties of various GH doses on both well-trained men and women. In the study, the “high-dose” group of subjects was getting the equivalent of ~20-25IUs of Humatrope, which is far higher than all but the most advanced bodybuilders would even dream of using. The most eye-opening aspect of the data presented was how differently males responded to IM versus SC dosing. The IM group of male subjects had a significantly higher GH peak despite having identical doses as the SC group. In fact, the IM male group peaked nearly as high as the high-dosed SC group despite having less than half the amount of GH injected. Conversely, the SC group had serum GH levels elevated for a longer overall duration though, which has been reported previously as well [32]. This is intriguing data; however there have been trials that demonstrate timelines associated with lipolysis occur in nearly an identical manner, independent of whether one uses IM or SC administration [18].
Following either SC or IM injections of GH, plasma FFA and glycerol levels increase after a brief lag period with rates of fat mobilization peaking around the 150-160 minute mark.
The other major point worth identifying is with regards to timing of the GH injection. The state of fasting is associated not only with a pronounced increase in endogenous GH secretion, as detailed earlier, but also increased lipolytic responsiveness to exogenous GH administration [33]. In fact, all lipolytic markers are enhanced when GH is administered in a fasted state as compared to being administered in the digestive/postprandial state [34]. The overall clearance rate is also significantly increased while staying in the fasted state.
It is certainly also worth noting that evening administration of GH has been shown to have a higher bioavailability, at least when provided to growth hormone deficient (GHD) subjects [35]. This could be due to it more closely mimicking the naturally-occurring endogenous evening release, but that is largely speculation at this point.
Compound Synergies
When designing a fat loss stack that maximizes lipolytic potential, it would serve us well to choose compounds with a potential synergy, or additive effects upon one another. Earlier, we discussed some of the high-level mechanisms by which GH mediates its lipolytic effects. One of its key pathways involves the beta-adrenergic receptors. So it would seem plausible that if we can increase beta-pathway sensitivity and/or expression, they we can further optimize the overall lipolytic response to our stack.
Androgens induce potent lipolytic effects directly via androgen receptors expressed in adipose tissues [36]. This is cool, in and of itself, because it is a different pathway than GH uses however androgens have also been shown to increase beta-adrenergic receptor expression [37–38]. I’ve already discussed how increasing the number of coupled receptors in adipocytes could increase sensitivity and ultimately lipolytic potential, so this could be a potent synergy in our stack design methodologies.
The thyroidal axis and the GH/IGF axis have a very interesting, albeit complicated, relationship with one another. Of particular interest to lipolytic stack design is its impacts on beta-adrenergic receptor mRNA and more specifically its impacts on β3 expression, which has been shown to be a critical step in GH-mediated lipolysis [39-41]. In addition thyroid hormones stimulate synthesis, degradation, and mobilization of lipids resulting in increased levels of circulating FFAs [42]. And the last synergistic characteristic worth mentioning is that they, just like GH, appear to regulate the sensitivity of metabolic processes to catecholamines.
And once you have that increased beta-adrenergic receptor sensitivity and density, it only stands to reason that a beta-adrenergic agonist would be the icing on the cake to fully maximize fat mobilization potential. Clenbuterol [43] is an excellent candidate for stack addition here; however a comprehensive review of beta-adrenergic agonists is beyond the scope of this article.
Practical Applications and Sample Stack Design
Now that we have all the background fodder out of the way, how would one go about designing a stack and administering their hormones?
I think it is pretty evident one will want to be fasted when dosing their GH. Although the lipolytic effects of rHGH are not entirely blunted in the presence of food (unlike endogenous GH), one would be doing a serious disservice by not adhering to this guideline.
Using the fasting guidelines, along with a dose designed to elicit maximum lipolytic response, would result in a single 2IU GH injection. Performing this upon waking in the AM would suit our needs very nicely since we would already be in the fasted state, more than likely. Because subcutaneous injections have a longer clearance time, this would be a good method to use for the injection, especially if planning to remain fasted for many hours following the injection. For a potential additive effect upon fat mobilization rates, I would also consider performing structured activity (either in the form of LISS or resistance training) during this fasted window [44].
Following the same guidelines, one could very likely administer a second 2IU injection of GH before going to bed, assuming they were able to consume all their meals in a condensed timeframe and enter the evening hours in a semi-fasted state. As illustrated earlier in the body of the article, there is even evidence that evening subcutaneous dosing provides higher bioavailability. So if one is forced to choose between AM and PM, this would be my recommended dosing time. If not, using a 2IU injection strategy both in the AM and PM can theoretically maximize one’s lipolytic potential over the course of 24 hours, assuming they adhere to the proper fasting guidelines.
Using this GH dosing protocol, in concert with an androgen stack, can enhance the overall potential for fat loss. One should consider supraphysiological AAS for this reason, as well as to prevent the risk of lean tissue atrophy during periods of sustained intake deficits. There may be minor differences in the rate of fat loss produced by using different androgen types, but this is beyond the scope of this article. Even a simple testosterone-based AAS stack design would work very nicely here.
Adding both exogenous thyroid and clenbuterol into the stack will further maximize the overall lipolytic potential based upon their synergistic properties with androgens and GH explained in the article. Due to their respective properties, clenbuterol can be dosed once per day as can T4. For those using T3, I recommend a minimum of two doses per day, spread 12 hours apart.
To bring it all together, in a simple to digest format, let’s provide a brief and high-level outline:
- Supraphysiological AAS stack
- Clenbuterol + Thyroid
- GH administered subcutaneously at a dose no greater than 2IUs in a fasted state (AM/PM) or PM if forced to choose
- Structured activity performed during the fasted state, post-injection to enhance fat mobilization potential
Editor’s Note
The article “The Most Effective Growth Hormone Protocol for Fat Loss” is a condensed and abbreviated excerpt from the GH section within the upcoming book “The Growth Hormone Handbook” (working title) by Chester “Chest” Rockwell.
References
- National Research Council (US) Committee on Technological Options to Improve the Nutritional Attributes of Animal Products. Designing Foods: Animal Product Options in the Marketplace. Washington (DC): National Academies Press (US); 1988. The Role of Growth Hormone in Fat Mobilization
- Lee, M. O., and Schaffer, N. K.: Anterior Pituitary Growth Hormone and the Composition of Growth , J. Nutrition 7: 337 ( (March 10) ) 1934.
- Li CH, Evans HM, Simpson ME. Isolation and properties of the anterior hypophyseal growth hormone. J Biol Chem. 1945;159:353–366.
- Schoemaker RC, Buijs MM, Pijl H, Burggraaf J, Cohen AF. Modeling the influence of growth hormone on lipolysis. J Pharmacokinet Pharmacodyn. 2002 Apr;29(2):157-70
- Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis – A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Progress in Lipid Research. 2011;50(1-4):14-27.
- ROTH J, GLICK SM, YALOW RS, BERSON SA. Secretion of human growth hormone:physiologic and experimental modification. Metabolism. 1963 Jul;12:577-9
- Hartman ML, Veldhuis JD, Johnson ML, Lee MM, Alberti KG, Samojlik E, Thorner MO. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J Clin Endocrinol Metab. 1992 Apr;74(4):757-65.
- Ho PJ, Friberg RD, Barkan AL. Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab. 1992 Sep;75(3):812-9.
- Goldenberg N, Barkan A. Factors regulating growth hormone secretion in humans. Endocrinol Metab Clin North Am. 2007 Mar;36(1):37-55. Review
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th edition. New York: W H Freeman; 2002. Section 30.3, Food Intake and Starvation Induce Metabolic Changes.
- Nørrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005 Apr;15(2):95-122. Review.
- Birnbaum MJ. Lipolysis: more than just a lipase. J Cell Biol. 2003 Jun 23;161(6):1011-2. Epub 2003 Jun 16. Review.
- Pedersen, S.B. & Børglum, Jens & Jorgensen, Jens & Richelsen, Bjørn. (1995). Growth hormone treatment of obese premenopausal women: Effects on isolated adipocyte metabolism. 2. 251-258.
- Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994 Sep;35(9):1535-41.
- Ottosson M, Vikman-Adolfsson K, Enerbäck S, Elander A, Björntorp P, Edén S. Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1995 Mar;80(3):936-41.
- Richelsen B. Effect of growth hormone on adipose tissue and skeletal muscle lipoprotein lipase activity in humans. J Endocrinol Invest. 1999;22(5 Suppl):10-5. Review.
- Richelsen B, Pedersen SB, Kristensen K, Børglum JD, Nørrelund H, Christiansen JS, Jørgensen JO. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000 Jul;49(7):906-11.
- Marcus C, Margery V, Kamel A, Brönnegård M. Effects of growth hormone on lipolysis in humans. Acta Paediatr Suppl. 1994 Dec;406:54-8; discussion 59. Review.
- Arner P, Hellmér J, Wennlund A, Ostman J, Engfeldt P. Adrenoceptor occupancy in isolated human fat cells and its relationship with lipolysis rate. Eur J Pharmacol. 1988 Jan 27;146(1):45-56
- Heffernan M, Summers RJ, Thorburn A, Ogru E, Gianello R, Jiang WJ, Ng FM. The effects of human GH and its lipolytic fragment (AOD9604) on lipid metabolism following chronic treatment in obese mice and beta(3)-AR knock-out mice. Endocrinology. 2001 Dec;142(12):5182-9.
- Hansen TK, Gravholt CH, ØRskov H, Rasmussen MH, Christiansen JS, Jørgensen JO. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J Clin Endocrinol Metab. 2002 Oct;87(10):4691-8
- Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol. 1991 Jan;260
- Haffner D, Schaefer F, Girard J, Ritz E, Mehls O 1994 Metabolic clearance of recombinant human growth hormone in health and chronic renal failure. J Clin Invest 93:1163–1171
- Fain JN. Effect of dibutyryl-3′,5′-AMP, theophylline and norepinephrine on lipolytic action of growth hormone and glucocorticoid in white fat cells. Endocrinology. 1968 Apr;82(4):825-30.
- Goodman HM. Effects of growth hormone on the lipolytic response of adipose tissue to theophylline. Endocrinology. 1968 May;82(5):1027-34.
- Yip RG, Goodman HM. Growth hormone and dexamethasone stimulate lipolysis and activate adenylyl cyclase in rat adipocytes by selectively shifting Gi alpha2 to lower density membrane fractions. Endocrinology. 1999 Mar;140(3):1219-27.
- Fain JN, Cheema P, Tichansky DS, Madan AK. Stimulation of human omental adipose tissue lipolysis by growth hormone plus dexamethasone. Mol Cell Endocrinol. 2008 Nov 25;295(1-2):101-5.
- Goodman HM 1970 Permissive effects of hormones on lipolysis. Endocrinology 86:1064–1074
- Davis E, Loiacono R, Summers RJ. The rush to adrenaline: drugs in sport acting on the β-adrenergic system. British Journal of Pharmacology. 2008;154(3):584-597
- Greenblatt, D. J. and Abourjaily, P. N. (2016), Pharmacokinetics and Pharmacodynamics for Medical Students: A Proposed Course Outline. The Journal of Clinical Pharmacology, 56: 1180–1195.
- Keller A, Wu Z, Kratzsch J, Keller E, Blum WF, Kniess A, Preiss R, Teichert J, Strasburger CJ, Bidlingmaier M. Pharmacokinetics and pharmacodynamics of GH:dependence on route and dosage of administration. Eur J Endocrinol. 2007 Jun;156(6):647-53
- Laursen T. Clinical pharmacological aspects of growth hormone administration. Growth Horm IGF Res. 2004 Feb;14(1):16-44. Review.
- Møller N, Pørksen N, Ovesen P, Alberti KG. Evidence for increased sensitivity of fuel mobilization to growth hormone during short-term fasting in humans. Horm Metab Res. 1993 Mar;25(3):175-9.
- Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, Jorgensen JO. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009 Mar;94(3):965-72
- Jørgensen JO, Møller N, Lauritzen T, Alberti KG, Orskov H, Christiansen JS. Evening versus morning injections of growth hormone (GH) in GH-deficient patients: effects on 24-hour patterns of circulating hormones and metabolites. J Clin Endocrinol Metab. 1990 Jan;70(1):207-14.
- Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009 Mar 25;301(1-2):97-103
- Xu X, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990 Feb;126(2):1229-34
- De Pergola G. The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord. 2000 Jun;24 Suppl 2:S59-63. Review
- Viguerie N, Millet L, Avizou S, Vidal H, Larrouy D, Langin D. Regulation of human adipocyte gene expression by thyroid hormone. J Clin Endocrinol Metab. 2002 Feb;87(2):630-4
- Rubio A, Raasmaja A, Silva JE. Thyroid hormone and norepinephrine signaling in brown adipose tissue. II: Differential effects of thyroid hormone on beta 3-adrenergic receptors in brown and white adipose tissue. Endocrinology. 1995 Aug;136(8):3277-84.
- Ghosh M, Das S. Increased beta(2)-adrenergic receptor activity by thyroid hormone possibly leads to differentiation and maturation of astrocytes in culture. Cell Mol Neurobiol. 2007 Dec;27(8):1007-21. Epub 2007 Sep 8
- Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000 Jun;24 Suppl 2:S109-12. Review
- Kim HK, Della-Fera MA, Hausman DB, Baile CA. Effect of clenbuterol on apoptosis, adipogenesis, and lipolysis in adipocytes. J Physiol Biochem. 2010 Sep;66(3):197-203
- Taaffe DR, Thompson JL, Butterfield GE, Hoffman AR, Marcus R. Recombinant human growth hormone, but not insulin-like growth factor-I, enhances central fat loss in postmenopausal women undergoing a diet and exercise program. Horm Metab Res. 2001 Mar;33(3):156-62.
Комментариев нет:
Отправить комментарий