"Concerta" redirects here. For the musical composition, see Concerto. For the implantable defibrillator named Medtronic Concerto, see defibrillator.
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
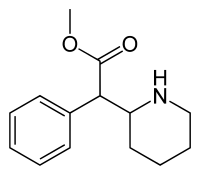
| methyl phenyl(piperidin-2-yl)acetate | |
| Clinical data | |
| Trade names | Concerta, Methylin, Ritalin, Equasym XL |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682188 |
| Licence data | US FDA:link |
| Pregnancy cat. | C |
| Legal status | Controlled (S8) (AU)Schedule III (CA) Class B(UK) Schedule II (US) |
| Dependence liability | Moderate |
| Routes | Oral, Transdermal |
| Pharmacokinetic data | |
| Bioavailability | 11–52% |
| Protein binding | 30% |
| Metabolism | Liver (80%) |
| Half-life | Immediate release tablets: 2 1/2 hours from oral take (2 hours from effect start);[1]extended-release capsules: 6 1/2 hours from oral take, 6 hours from effect start or additional hour for some release systems[citation needed] |
| Excretion | Urine |
| Identifiers | |
| CAS number | 113-45-1 |
| ATC code | N06BA04 |
| PubChem | CID 4158 |
| DrugBank | DB00422 |
| ChemSpider | 4015 |
| UNII | 207ZZ9QZ49 |
| KEGG | D04999 |
| ChEBI | CHEBI:6887 |
| ChEMBL | CHEMBL796 |
| Chemical data | |
| Formula | C14H19NO2 |
| Mol. mass | 233.30 g/mol |
| Physical data | |
| Melt. point | 214 °C (417 °F) |
| | |
Methylphenidate is a psychostimulant drug and substituted phenethylamine approved for treatment of attention-deficit hyperactivity disorder (ADHD), postural orthostatic tachycardia syndrome and narcolepsy. The original patent was owned by CIBA, now Novartis Corporation. It was first licensed by the U.S. Food and Drug Administration (FDA) in 1955 for treating what was then known as hyperactivity. Prescribed to patients beginning in 1960, the drug became heavily prescribed in the 1990s, when the diagnosis of ADHD itself became more widely accepted.[2]
ADHD and other similar conditions are believed to be linked to sub-performance of the dopamine and norepinephrine functions in the brain, primarily in the prefrontal cortex, responsible for self-regulation functions of inhibition, motivation, memory, and the concentration/executive functions of reasoning, organizing, solving, and planning.[3][4] Methylphenidate's pharmacological profile involves catecholamines, similar to other sympathomimetics of the phenethylamine class. In particular, methylphenidate is adopamine reuptake inhibitor and also a much weaker norepinephrine reuptake inhibitor, which increases the levels of theseneurotransmitters in the brain.
Contents
[hide]Medical uses[edit]
MPH is the most commonly prescribed psychostimulant and works by increasing the activity of the central nervous system.[5] It produces such effects as increasing or maintaining alertness, combating fatigue, and improving attention.[6] The short-term benefits and cost effectiveness of methylphenidate are well established, although long-term effects are unknown.[7][8] The long term effects of methylphenidate on the developing brain are unknown. Methylphenidate is not approved for children under six years of age.[9][10]Methylphenidate may also be prescribed for off-label use in treatment-resistant cases of lethargy, bipolar disorder, major depressive disorder, and obesity.[citation needed]
Attention deficit hyperactivity disorder[edit]
Methylphenidate is approved by the U.S. Food and Drug Administration (FDA) for the treatment of attention deficit hyperactivity disorder.[11] The addition of behavioural modification therapy (e.g. cognitive behavioral therapy (CBT)) has additional benefits on treatment outcome.[12][13] People with ADHD have an increased risk of substance abuse, and stimulant medications reduce this risk.[14][15] A meta analysis of the literature concluded that methylphenidate quickly and effectively reduces the signs and symptoms of ADHD in children under the age of 18 in the short term but found that this conclusion may be biased due to the high number of low quality clinical trials in the literature.[citation needed]
Methylphenidate's long-term efficacy in ADHD treatment has been questioned because of a lack of long-term studies and possiblepublication bias.[16] A 2010 study suggested that, "there is increasing evidence...[stimulant drugs such as methylphenidate] do not promote learning and academic achievement".[17]
Some research suggests that methylphenidate treatment should not be indefinite. Weaning off periods to assess symptoms and allay tolerance are recommended.[18]
The dosage used can vary quite significantly from individual child to individual child with some children responding to quite low doses whereas other children require the higher dose range. The dose, therefore, should be titrated to an optimal level that achieves therapeutic benefit and minimal side-effects.[19] This can range from anywhere between 5–30 mg twice daily or up to 60 mg a day.
Mechanisms of ADHD[edit]
Main article: ADHD#Cause
The means by which methylphenidate affects people diagnosed with ADHD are not well understood. Some researchers have theorized that ADHD is caused by a dopamineimbalance in the brains of those affected. Methylphenidate is a norepinephrine and dopamine reuptake inhibitor, which means that it increases the level of the dopamineneurotransmitter in the brain by partially blocking the dopamine transporter (DAT) that removes dopamine from the synapses.[20] This inhibition of DAT blocks the reuptake of dopamine and norepinephrine into the presynaptic neuron, increasing the amount of dopamine in the synapse. It also stimulates the release of dopamine and norepinephrine into the synapse. Finally, it increases the magnitude of dopamine release after a stimulus, increasing the salience of stimulus. An alternate explanation that has been explored is that the methylphenidate affects the action of serotonin in the brain.[21][22] However, benefits with other stimulants that have a different mechanism of action indicates that support for a deficit in specific neurotransmitters is unsupported and unproven by the evidence and remains a speculative hypothesis.[23]
Narcolepsy[edit]
Narcolepsy, a chronic sleep disorder characterized by overwhelming daytime drowsiness and sudden need for sleep, is treated primarily with stimulants. Methylphenidate is considered effective in increasing wakefulness, vigilance, and performance.[24] Methylphenidate improves measures of somnolence on standardized tests, such as the Multiple Sleep Latency Test, but performance does not improve to levels comparable to healthy controls.[25]
Aggression and criminality[edit]
Newer studies indicate methylphenidate in the treatment of ADHD in adults with a history of aggressive and criminal behavior. A large clinical study conducted in Sweden found a significant reduction of the criminality rate in males (32%) and females (42%) as compared with the rate for the same patients while not receiving medication.[26] Some of these clinical outcomes have been confirmed in similar studies with children and adolescents.[27]
Adjunctive[edit]
Use of stimulants such as methylphenidate in cases of treatment resistant depression is controversial.[28] In individuals with cancer, methylphenidate is commonly used to counteract opioid-induced somnolence, to increase the analgesic effects of opioids, to treat depression, and to improve cognitive function.[29] Methylphenidate may be used in addition to an antidepressant for refractory major depressive disorder. It can also improve depression in several groups including stroke, cancer, and HIV-positive patients.[30]However, benefits tend to be only partial with stimulants. Stimulants may however, have fewer side-effects than tricyclic antidepressants in the elderly and medically ill.[31]
Substance dependence[edit]
Although possible, substance dependence is rare with Methylphenidate.[32] Methylphenidate has shown some benefits as a replacement therapy for individuals dependent onmethamphetamine.[33] Methylphenidate and amphetamine have been investigated as a chemical replacement for the treatment of cocaine dependence[34][35][36][37] in the same way that methadone is used as a replacement for heroin. Its effectiveness in treatment of cocaine or psychostimulant dependence has not been proven and further research is needed.[38]
Early research began in 2007–2008 by Pharmacokinetics and Biopharmaceutics Laboratory, Department of Pharmaceutical Sciences, School of Pharmacy, in University of Maryland, Baltimore, Maryland, first published, 19 September 2007 in the United States[39] on the effectiveness of methylphenidate as a substitute agent in refractory cases of cocaine dependence.[40][41]
Investigational[edit]
Animal studies using rats with ADHD-like behaviours were used to assess the safety of methylphenidate on the developing brain and found that psychomotor impairments, structural and functional parameters of the dopaminergic system were improved with treatment. This animal data suggests that methylphenidate supports brain development and hyperactivity in children diagnosed with ADHD. However, in normal control animals methylphenidate caused long lasting changes to the dopaminergic system suggesting that if a child is misdiagnosed with ADHD they may be at risk of long lasting adverse effects to brain development. Animal tests found that rats given methylphenidate grew up to be more stressed and emotional. It is unclear due to lack of follow-up study whether this occurs in ADHD like animals and whether it occurs in humans.[42] However, long lasting benefits of stimulant drugs have not been found in humans.[43]
Adverse effects[edit]
Some adverse effects may emerge during chronic use of methylphenidate so a constant watch for adverse effects is recommended.[44] Some adverse effects of stimulant therapy may emerge during long-term therapy, but there is very little research of the long-term effects of stimulants.[45][46] The most common side effects of methylphenidate are nervousness, drowsiness and insomnia.[citation needed] Other adverse reactions include:[47]
- Abdominal pain
- Akathisia (restlessness)
- Alopecia (loss of hair)
- Angina (chest pain)
- Appetite loss
- Anxiety/Nervousness
- Blood pressure and pulse changes (both up and down)
- Cardiac arrhythmia
- Depression
- Diaphoresis (sweating)
- Dizziness
- Dyskinesia (uncontrollable tics)
- Euphoria or dysphoria
- Headache
- Hypersensitivity (including skin rash, urticaria, fever, arthralgia, exfoliative dermatitis,erythema multiforme, necrotizing vasculitis, and thrombocytopenic purpura)
- Irritability
- Lethargy
- Libido increased or decreased
- Mania or hypomania
- Nausea
- Palpitations
- Pupil dilation[48]
- Psychosis and psychiatric disorders - stimulants above the recommended dose level are associated with higher levels of psychosis, substance misuse and psychiatric admissions.[49]
- Short-term weight loss
- Somnolence
- Stunted growth
- Suicidal ideation
- Tachycardia (rapid resting heart rate)
- Xerostomia (dry mouth)
On March 22, 2006, the FDA Pediatric Advisory Committee decided that medications using methylphenidate ingredients do not need black box warnings about their risks, noting that "for normal children, these drugs do not appear to pose an obvious cardiovascular risk."[50] Previously, 19 possible cases had been reported of cardiac arrest linked to children taking methylphenidate[51] and the Drug Safety and Risk Management Advisory Committee to the FDA recommend a "black-box" warning in 2006 for stimulant drugs used to treat attention deficit/hyperactivity disorder.[52]
Historical concerns related to child growth and cancer risk have existed, and these are still monitored and studied, however current scientific consensus is that the evidence of studies suggests these are either dubious or low-significance risks. (See : Previous health concerns now considered doubtful or largely minor)
Treatment emergent psychosis[edit]
Main article: Stimulant psychosis
On occasion, treatment emergent psychosis can occur during long-term therapy with methylphenidate. Regular psychiatric monitoring of people who are taking methylphenidate for adverse effects such as psychotic symptomatology has been recommended.[53] In the majority of unremarkable isolated cases methylphenidate overdose is asymptomatic or only incurs minor symptoms even in children under six years of age.[54][55][56] Normally any reaction will show within three hours.[56] However, injection (particularly arterial) has sometimes led to toxic necrosis and amputation at the point of injection.[57] Emergency treatment is recommended beyond certain overdose levels, in cases of attempted suicide, and in those using monoamine oxidase inhibitors (MAOIs).[56]
Long-term effects[edit]
In 2000, by Zito et al.[58] documented "that at least 1.5% of children between the ages of two and four are medicated with stimulants, anti-depressants and anti-psychotic drugs, despite the paucity of controlled scientific trials confirming safety and long-term effects with preschool children."
The effects of long-term methylphenidate treatment on the developing brains of children with ADHD is the subject of study and debate.[59][60] Although the safety profile of short-term methylphenidate therapy in clinical trials has been well established, repeated use of psychostimulants such as methylphenidate is less clear. There are no well defined withdrawal schedules for discontinuing long-term use of stimulants.[61] There is limited data that suggests there are benefits to long-term treatment in correctly diagnosed children with ADHD, with overall modest risks.[62] Short-term clinical trials lasting a few weeks show an incidence of psychosis of about 0.1%.[63] A small study of just under 100 children that assessed long-term outcome of stimulant use found that 6% of children became psychotic after months or years of stimulant therapy. Typically, psychosis would abate soon after stopping stimulant therapy. As the study size was small, larger studies have been recommended.[64] The long-term effects on mental health disorders in later life of chronic use of methylphenidate is unknown.[65] Concerns have been raised that long-term therapy might cause drug dependence, paranoia, schizophrenia and behavioral sensitisation, similar to other stimulants.[66] Psychotic symptoms from methylphenidate can include hearing voices[citation needed], visual hallucinations[citation needed], urges to harm oneself[citation needed], severe anxiety[citation needed], euphoria[citation needed], grandiosity[citation needed], paranoid delusions[citation needed], confusion[citation needed], increasedaggression[citation needed] and irritability[citation needed]. Methylphenidate psychosis is unpredictable in whom it will occur. Family history of mental illness does not predict the incidence of stimulant toxicosis in children with ADHD[citation needed]. High rates of childhood stimulant use is found in patients with a diagnosis of schizophrenia and bipolar disorder independent of ADHD[citation needed]. Individuals with a diagnosis of bipolar or schizophrenia who were prescribed stimulants during childhood typically have a significantly earlier onset of the psychotic disorder and suffer a more severe clinical course of psychotic disorder.[dubious ][67][68][69]
Knowledge of the effects of chronic use of methylphenidate is poorly understood with regard to persisting behavioral and neuroadaptational effects.[70] Juvenile rhesus monkeys chronically administered twice daily methylphenidate doses that cause plasma levels similar to those of higher pharmalogical doses in humans show no apparent lasting effects.[71]Measures tested included D2-like dopamine receptor density, dopamine transporter density, amphetamine-induced dopamine release responsiveness, cognitive performance, and growth.[71]
Precautions[edit]
Interactions[edit]
Intake of adrenergic agonist drugs or pemoline with methylphenidate increases the risk of liver toxicity.[72][73] When methylphenidate is coingested with ethanol, a metabolite calledethylphenidate is formed via hepatic transesterification,[74][75] not unlike the hepatic formation of cocaethylene from cocaine and alcohol. The reduced potency of ethylyphenidate and its minor formation means it does not contribute to the pharmacological profile at therapeutic doses and even in overdose cases ethylphenidate concentrations remain negligible.[5][76] Coingestion of alcohol (ethanol) also increases the blood plasma levels of d-methylphenidate by up to 40%.[77] Ethylphenidate is more selective to the dopamine transporter (DAT) than methylphenidate, having approximately the same efficacy as the parent compound,[78] but has significantly less activity on the norepinephrine transporter(NET).[79]
Contraindications[edit]
Methylphenidate should not be prescribed concomitantly with tricyclic antidepressants, such as desipramine, or monoamine oxidase inhibitors, such as phenelzine ortranylcypromine, as methylphenidate may dangerously increase plasma concentrations, leading to potential toxic reactions (mainly, cardiovasculareffects).[medical citation needed][vague] Methylphenidate should not be prescribed to patients who suffer from severe arrhythmia, hypertension or liver damage. It should not be prescribed to patients who demonstrate drug-seeking behaviour, pronounced agitation or nervousness.[18] Care should be taken while prescribing methylphenidate to children with a family history of Paroxysmal Supraventricular Tachycardia (PSVT).
Special precautions[edit]
Special precaution is recommended in individuals with epilepsy with additional caution in individuals with uncontrolled epilepsy due to the potential for methylphenidate to lower the seizure threshold.[80] There is no published evidence to suggest that either the short or long term treatment with methylphenidate increases the risk of developing seizures in children with ADHD.[80] A number of small trials suggest that it is safe for use in children with epilepsy. Further randomised control trials are needed.
Pregnancy[edit]
The U.S. FDA gives methylphenidate a pregnancy category of C, and women are advised to only use the drug if the benefits outweigh the potential risks.[81] Not enough animal and human studies have been conducted to conclusively demonstrate an effect of methylphenidate on fetal development. In 2007, empirical literature included 63 cases of prenatal exposure to methylphenidate across three empirical studies.[82] One of these studies (N = 11) demonstrated no significant increases in malformations.[83] A second (N = 13) demonstrated one major malformation in newborns with early exposure to methylphenidate, which was in the expected range of malformations. However, this was a cardiac malformation, which was not within the statistically expected range.[84] Finally, in a retrospective analysis of patients' medical charts (N = 38), researchers examined the relationship between abuse of intravenous methylphenidate and pentazocine in pregnant women. Twenty-one percent of these children were born prematurely, and several had stunted growth and withdrawal symptoms (31% and 28%, respectively). Intravenous methylphenidate abuse was confounded with the concurrent use of other substances (e.g.,cigarettes, alcohol) during pregnancy.
Overdose and toxicology[edit]
In the majority of unremarkable isolated cases MPH overdose is asymptomatic (symptomless) or only incurs minor symptoms even in children under age 6.[54][55][56] In cases that manifest symptoms, these can typically include agitation, hallucinations, psychosis, lethargy, seizures, tachycardia, dysrhythmias, hypertension, and hyperthermia.[85] LD50 in mice is 190 mg/kg.[86]
Studies of reported incidents tend to show that most overdoses are unintentional and generally conclude that severe or major toxicity are comparatively rare events (none in the Michigan study of 289 incidents,[54] 0.9% in the 2004 US national analysis with n=8336,[87] and 0.2% in the same analysis for 2010 with n=6503[55]).
Death rates are also comparatively low (none in the Michigan study, 0.36 per 1000 with n=3 for the 2004 US national analysis, 0.15 per 1000 with n=1 for the 2010 analysis; the US national guideline approved 2007 also notes only 2 deaths reported as primarily to MPH overdose from 2000-05[56]).
A 2008 review generally agreed these findings but noted recreation or study use was "fairly common" in US university studies and that the risk could only be said to be low "in the short term" since there was little certainty about long term effects of overdose and abuse.[88] A 2011 Swiss study also agreed the general findings, adding a cautionary note that serious or severe outcomes such as necrosis, abscess and amputation had occurred as a result of severe toxicity at the injection site in 3 cases of abuse via injection, especially when arterial.[57]
Medical and emergency handling[edit]
Key recommendations in US guidelines for overdose handling include:[56]
- Well evidenced findings (evidence standard "A"): 0–6 years: <2 mg/kg rarely causes serious toxicity, 0–5 years: up to 40 mg well tolerated, 6–12 years: up to 80 mg well tolerated;
- Evidence grade "B" and "C": If <6 years and >2 mg/kg, or <60 kg and >1 mg/kg, or ≥60 kg and >60 mg: refer to emergency help;
- Tentative only (D): 4 mg/kg or 120 mg of intact modified (slow) release version: refer to emergency help.
- Symptoms (D): "Patients experiencing any changes in behavior other than mild stimulation or agitation should be referred to an emergency department. Examples of moderate to severe symptoms that warrant referral include moderate-to-severe agitation, hallucinations, abnormal muscle movements, headache, chest pain, loss of consciousness, or convulsions".
- Other factors: Cases of intent, malicious administration (by another), as well as monoamine oxidase inhibitor (MAOI) users should always be referred to emergency help;
- Passage of time/delay: Patients where more than 3 hours have passed without symptoms do not usually need referral to emergency help.
- Benzodiazepines may be used as treatment if agitation, dystonia, or convulsions are present.
Poison control centre analyses and study findings[edit]
A study in 2000 looked in detail at all 289 overdoses of MPH reported to the Children's Hospital of Michigan regional poison control center during 1993 and 1994 (excluded: 105 extended-release formulations or co-ingestants, to ensure MPH overdose effects were not confounded by other effects).[54] The case histories were: Age: 251 aged under 18, 38 adult; Reason: 68 (23%) intentional/unknown/error. In 163 cases (56%) the dose was known and in 41% the patient's own MPH was involved. Variation in overdose ranged from <1 mg/kg (30%) to >3 mg/kg (7.5%) mean 1.7 mg/kg. Findings:
- Although no patient developed "severe" symptoms, but "less favourable" symptoms were seen with intentional overdoses. In overdoses below 2 mg/kg the majority (63-75%) suffered no effect and a minority (9-16%) suffered a moderate effect. Above 3 mg/kg around 27% suffered a moderate effect. Overall symptoms occurred in 31% of all overdoses. In paediatric exposures 29% developed symptoms but 66% suffered no clinical effects (mild/moderate effects: 34%). Symptomatic findings were:[54]
- "Intentional ingestion of MPH was most commonly associated with isolated symptoms of tachycardia, agitation, lethargy, vomiting, dizziness, mydriasis, and tremor. Of the 8 patients in this group who manifested multiple symptoms, erythema, diaphoresis, hypertension, emesis, chest pain, tremor, fever, and insomnia"
- Symptoms were common (33%) in the 0-5 age group: "Isolated lethargy, agitation, headache, and vomiting were most commonly seen. One patient in this group developed dystonia, and two developed agitation in combination with hypertension or tachycardia."
In 2004, the American Association of Poison Control Centers Toxic Exposure Surveillance System annual report showed about 8300 methylphenidate ingestions reported in US poison center data,[56][87] of which 72% were accidental or unintended, and 19% involved children age 0-6. The most common reasons for intentional exposure were drug abuse and suicide attempts.[89] The 2010 report[55] showed 6500 single reported exposures in the US for the year. 2010 incidents:
- By age: 0-5: 24%, 6-12: 38%, 13-19:21%, 20+: 16%, other adult: 1%.
- By cause: accident/error: 79%, intended: 18%, other: 3%.
- By outcome: moderate: 624, major:13, death:1, others were no outcome, minor, or unknown. (2004 outcomes: moderate: 940, major: 73, death: 3)[87]
A Swiss study in 2011 also concurred, noting similar findings in several studies and national analyses in that country, but noted that these findings were potentially inapplicable to the few cases of abuse via crushed MPH injection, which was the sole situation where "serious" or "severe" local toxicity was observed, leading in their study to pain, necrosis and partial limb or digit amputation in two of 14 adult cases over 8 years (14%) who mistakenly injected arterially, and inguinal abscess and fever in one who injected intravenously.[57]
Tolerance[edit]
Tolerance and behavioural sensitisation may occur with long-term use of methylphenidate.[77] There is also cross tolerance with other stimulants, such as amphetamine.[90]Stimulant withdrawal or rebound reactions can occur and should be minimised in intensity, e.g. via a gradual tapering off of medication over a period of weeks or months.[91][92][93]A very small study of abrupt withdrawal of stimulants did suggest that withdrawal reactions are not typical. Nonetheless, withdrawal reactions may still occur in susceptible individuals.[94] The withdrawal or rebound symptoms of methylphenidate can include psychosis, depression, irritability and a temporary worsening of the original ADHD symptoms. Methylphenidate, due to its very short elimination half life, may be more prone to rebound effects than d-amphetamine.[18][95][96] Up to a third of children with ADHD experience arebound effect when methylphenidate dose wears off.[97]
However, there have also been studies that show that chronic administration of methylphenidate increases sensitivity.[98] This phenomenon, known as sensitization, is known to occur with chronic administration of amphetamine.[99]
Abuse potential[edit]
Methylphenidate has some potential for abuse due to its action on dopamine transporters. Methylphenidate, like other stimulants, increases dopamine levels in the brain, but at therapeutic doses this increase is slow, and thus euphoria only rarely occurs even when it is administered intravenously.[100] The abuse and addiction potential of methylphenidate is therefore significantly lower than other dopaminergic stimulants.[100][101] The abuse potential is increased when methylphenidate is crushed and insufflated (snorted), or injected.[102] However, the dose that produces euphoric effects varies among individuals. The primary source of methylphenidate for abuse is diversion from legitimate prescriptions, rather than illicit synthesis. Those who use methylphenidate medicinally generally take it orally, while intranasal and intravenous are the preferred means for recreational use.[85] IV users tend to be adults whose use may cause panlobular pulmonary emphysema.[89]
Abuse of prescription stimulants is higher amongst college students than non-college attending young adults. College students use methylphenidate either as a study aid or to stay awake longer. Increased alcohol consumption due to stimulant misuse has additional negative effects on health.[103]
Patients who have been prescribed Ritalin have been known to sell their tablets to others who wish to take the drug recreationally[citation needed]. In the USA it is one of the top ten stolen prescription drugs.[medical citation needed] Recreational users may crush the tablets and either snort the powder, or dissolve the powder in water, filter it through cotton wool into a syringe to remove the inactive ingredients and other particles and inject the drug intravenously[citation needed]. Both of these methods increase bioavailability and produce a much more rapid onset of effects than when taken orally (within c. 5–10 minutes through insufflation and within just 10–15 seconds through intravenous injection); however the overall duration of action tends to be decreased by any non-oral use of drug preparations made for oral use.[104]
Methylphenidate is sometimes used by students to enhance their mental abilities, improving their concentration and helping them to study. Professor John Harris, an expert in bioethics, has said that it would be unethical to stop healthy people taking the drug. He also argues that it would be "not rational" and against human enhancement to not use the drug to improve people's cognitive abilities.[105] Professor Anjan Chatterjee however has warned that there is a high potential for abuse and may cause serious adverse effects on the heart, meaning that only people with an illness should take the drug. In the British Medical Journal he wrote that it was premature to endorse the use of Ritalin in this way as the effects of the drug on healthy people have not been studied.[106][107] Professor Barbara Sahakian has argued that the use of Ritalin in this way may give students an unfair advantage in examinations and that as a result universities may want to discuss making students give urine samples to be tested for the drug.[108]
Legal status[edit]
- Internationally, methylphenidate is a Schedule II drug under the Convention on Psychotropic Substances.[109]
- In the United States, methylphenidate is classified as a Schedule II controlled substance, the designation used for substances that have a recognized medical value but present a high potential for abuse.
- In the United Kingdom, methylphenidate is a controlled 'Class B' substance. Possession without prescription carries with a sentence up to 5 years and/or an unlimited fine, and supplying it is 14 years and/or an unlimited fine.[110]
- In Canada, methylphenidate is listed in Schedule III of the Controlled Drugs and Substances Act (along with LSD, psychedelic mushrooms, and mescaline, among others), and is illegal to possess without a prescription, pursuant to Part G (section G.01.002) of the Food and Drug Regulations under the Food and Drugs Act.
- In New Zealand, methylphenidate is a 'class B2 controlled substance'. Unlawful possession is punishable by six-month prison sentence and distribution of it is punishable by a 14-year sentence.
- In Australia, methylphenidate is a 'Schedule 8' controlled substance. Such drugs must be kept in a lockable safe before being handed out and possession without prescription carries hefty fines and even imprisonment.
Available forms[edit]
The dosage forms of methylphenidate are tablets, capsules, patches, and liquid.
Immediate-release[edit]
A formulation by the Novartis trademark name Ritalin, is an immediate-release racemic mixture, although a variety of formulations and generic brand names exist. Generic brand names include Ritalina, Rilatine, Attenta, Medikinet, Metadate, Methylin, Penid, and Rubifen. Focalin is a preparation containing only dextro-methylphenidate, rather than the usual racemic dextro- and levo-methylphenidate mixture of other formulations.
Extended-release[edit]
Extended-release tablets or capsules include:
- Concerta (brand-name); Watson methylphenidate ER (US generic); Teva-Methylphenidate ER‑C (Canadian generic). Each pill is effective for 12 hours.[111]
- Equasym XL; Medikinet XL; Metadate CD; Ritalin LA; Rubifen SR. Some of these work identically to each other; some do not.
- Ritalin‑SR (brand-name); Methylin ER (US generic); Metadate ER (US generic); methylphenidate SR (Canadian generic).[112][113][114]Each pill is effective for 5–8 hours.[111]
A newer way of taking methylphenidate is by using a transdermal patch (under the brand name Daytrana), similar to those used fornicotine replacement therapy.
Concerta tablets are marked with the letters "ALZA" and followed by: "18", "27", "36", or "54", relating to the mg dosage strength. Approximately 22% of the dose is immediate release,[115] and the remaining 78% of the dose is released over 10–12 hours post ingestion, with an initial increase over the first 6 to 7 hours, and subsequent decline in released drug.[116]
Ritalin LA capsules are marked with the letters "NVR" (abbrev.: Novartis) and followed by: "R20", "R30", or "R40", depending on the (mg) dosage strength. Both Ritalin LA[117] and Equasym XL provide two standard doses – half the total dose being released immediately and the other half released four hours later. In total, each capsule is effective for about eight hours.
Metadate CD capsules contain two types of beads; 30% of the beads are immediate release, and the other 70% of the beads are evenly sustained release.[118]
Controversy[edit]
Main article: Attention deficit hyperactivity disorder controversies
Methylphenidate has been the subject of controversy in relation to its use in the treatment of ADHD. One such criticism is prescribing psychostimulants medication to children to reduce ADHD symptoms.[119] The contention that methylphenidate acts as a gateway drug has been discredited by multiple sources,[120] according to which abuse is statistically very low and "stimulant therapy in childhood does not increase the risk for subsequent drug and alcohol abuse disorders later in life".[121]
Another controversial idea surrounding ADHD is whether to call it a disorder when patients, in general, have healthy appearing brains with no gross neurological deficits.[122]
Treatment of ADHD by way of Methylphenidate has led to legal actions including malpractice suits regarding informed consent, inadequate information on side effects,misdiagnosis, and coercive use of medications by school systems.[123] In the U.S. and the United Kingdom, it is approved for use in children and adolescents. In the U.S., theFood and Drug Administration approved the use of methylphenidate in 2008 for use in treating adult ADHD.[124] Methylphenidate has been approved for adult use in the treatment ofnarcolepsy.[125]
The pharmacological effects of methylphenidate resemble those of the class of DNRIs,[126] which is useful in the treatment of ADHD.[127]
Shortages of Ritalin in 2011[128] have been blamed on overmedication, itself ironically due to inattention to alternative therapies or measurement of long-term efficacy.[129]Attempts have been made to rebut these charges, primarily by questioning the assumptions of studies conducted long after the treatment period has ended.[130]
Chemistry[edit]
Four isomers of methylphenidate are known to exist. One pair of threo isomers and one pair of erythro are distinguished, from which only d-threo-methylphenidate exhibits the pharmacologically usually desired effects.[131][132] When the drug was first introduced it was sold as a 3:1 mixture of erythro:threo diastereomers. The erythro diastereomers are also pressor amines. "TMP" is referring only to the threo product that does not contain any erythro diastereomers. Since the threo isomers are energetically favored, it is easy toepimerize out any of the undesired erythro isomers. The drug that contains only dextrorotary methylphenidate is called d-TMP. A review on the synthesis of enantiomerically pure (2R,2'R)-(+)-threo-methylphenidate hydrochloride has been published.[133]
Production and brand-names[edit]
Methylphenidate is produced in the United States, Mexico, Spain and Pakistan. Ritalin is also sold in Canada, Australia, the United Kingdom, Spain, Germany and other European countries (although in much lower volumes than in the United States). Other brands include Concerta, Methylin, and Daytrana, and generic forms, including Methylin,Metadateand Attenta are produced by numerous pharmaceutical companies throughout the world. In Belgium the product is sold under the name Rilatine and in Brazil, Portugal and Argentina as Ritalina. In Thailand, it is found under the name Hynidate.
The dextrorotary enantiomer of methylphenidate, known as dexmethylphenidate, is sold as a generic and under the brand names Focalin and Attenade.
History[edit]
Methylphenidate was synthesized by Ciba (now Novartis) chemist Leandro Panizzon. His wife, Marguerite, had low blood pressure and would take the drug as a stimulant before playing tennis. He named the substance Ritaline, after his wife's nickname, Rita.[138]
Originally it was marketed as a mixture of two racemates, 80% (±)-erythro and 20% (±)-threo. Subsequent studies of the racemates showed that the central stimulant activity is associated with the threo racemate and were focused on the separation and interconversion of the erythro isomer into the more active threo isomer.[139][140][141]
Beginning in the 1960s, it was used to treat children with ADHD or ADD, known at the time as hyperactivity or minimal brain dysfunction (MBD). Production and prescription of methylphenidate rose significantly in the 1990s, especially in the United States, as the ADHD diagnosis came to be better understood and more generally accepted within the medical and mental health communities.[142]
In 2000 Janssen received U.S. Food and Drug Administration (FDA) approval to market "Concerta", an extended-release form of Ritalin.[143] See the "Extended-release" section of this article, below, for more information about Concerta.
Pharmacology[edit]
Methylphenidate primarily acts as a dopamine-norepinephrine reuptake inhibitor. It is a benzylpiperidine and phenethylaminederivative which also shares part of its basic structure with catecholamines.
Methylphenidate is most active at modulating levels of dopamine and to a lesser extent norepinephrine.[131] Methylphenidate binds to and blocks dopamine transporters and norepinephrine transporters.[144]
While both amphetamine and methylphenidate are dopaminergic, it should be noted that their methods of action are distinct. Specifically, methylphenidate is a dopamine reuptake inhibitor while amphetamine is both a releasing agent and reuptake inhibitor of dopamine and norepinephrine. Each of these drugs has a corresponding effect on norepinephrine which is weaker than its effect on dopamine. Methylphenidate's mechanism of action at dopamine-norepinephrine release is still debated, but is fundamentally different from most other phenethylamine derivatives, as methylphenidate is thought to increase general firing rate, whereasamphetamine reverses the flow of the monoamine transporters via TAAR1.[21][145][146][147][148] Moreover, MPH is thought to act as a releasing agent by increasing the release of dopamine and norepinephrine, though to a much lesser extent than amphetamine.[149]
Methylphenidate has both dopamine transporter and norepinephrine transporter binding affinity, with the dextromethylphenidateenantiomers displaying a prominent affinity for the norepinephrine transporter. Both the dextrorotary and levorotary enantiomers displayed receptor affinity for the serotonergic 5HT1A and 5HT2B subtypes, though direct binding to the serotonin transporter was not observed.[150]
Methylphenidate may also exert a neuroprotective action against the neurotoxic effects of Parkinson's disease and methamphetamine abuse.[151]
The dextrorotary enantiomers are significantly more potent than the levorotary enantiomers, and some medications therefore only contain dexmethylphenidate.[131]
ADHD and stimulant dynamics in general[edit]
Main article: Attention deficit hyperactivity disorder#Pathophysiology
Studies confirm that biological and genetic differences of the kinds predicted by low arousal theory are clearly visible in ADHD sufferers, and have been confirmed both genetically and by in vivo scans of ADHD affected brains. MRI scans have revealed that people with ADHD show differences from non-ADHD individuals in brain regions important for attention regulation and control of impulsive behavior.[152] Methylphenidate's cognitive enhancement effects have been investigated using fMRI scans even in non-ADHD brains, which revealed modulation of brain activity in ways that enhance mental focus. Methylphenidate increases activity in the prefrontal cortex and attention-related areas of the parietal cortex during challenging mental tasks; these are the same areas that the above study demonstrated to be shrunken in ADHD brains. Methylphenidate also increased deactivation of default network regions during the task.[153]
Pharmacokinetics[edit]
Methylphenidate taken orally has a bioavailability of 11-52% with a duration of peak action around 2–4 hours for instant release, 3–8 hours for sustained release, and 8–12 hours for extended release (Concerta). The half-life of methylphenidate is 2–3 hours, depending on the individual. The peak plasma time is achieved at about 2 hours.[154] Contrary to the expectation, taking methylphenidate with a meal speeds absorption.[155]
Detection in biological fluids[edit]
The concentration of methylphenidate or ritalinic acid, its major metabolite, may be quantified in plasma, serum or whole blood in order to monitor compliance in those receiving the drug therapeutically, to confirm the diagnosis in potential poisoning victims or to assist in the forensic investigation in a case of fatal overdosage.[156]
References[edit]
- ^ Kimko, H. C.; Cross, J. T.; Abernethy, D. R. (1999). "Pharmacokinetics and clinical effectiveness of methylphenidate". Clinical pharmacokinetics 37 (6): 457–470.doi:10.2165/00003088-199937060-00002. PMID 10628897.
- ^ www.ehow.com/about_5374709_ritalin-invented.html When Was Ritalin Invented?, citing Lawrence Diller: "Running on Ritalin", 1999
- ^ "Functional Roles of Norepinephrine and Dopamine in ADHD: Dopamine in ADHD".Medscape. 2006. Retrieved 2013-10-08. "Catecholamines not only facilitate attention, they are essential to executive function. The prefrontal cortex directs behaviors, thoughts, and feelings represented in working memory. This representational knowledge is essential to fundamental cognitive abilities that compromise executive functions. These encompass the ability to (1) inhibit inappropriate behaviors and thoughts, (2) regulate our attention, (3) monitor our actions, and (4) plan and organize for the future. Difficulties with these prefrontal cortex functions are evident in neuropsychological and imaging studies of ADHD patients and account for many of the common behavioral symptoms. Measures of prefrontal cortical functioning in animals indicate that these functions are sensitive to small changes in catecholamine modulation of prefrontal cortex cells that can produce profound effects on the ability of the prefrontal cortex to guide behavior. Optimal levels of NE acting at postsynaptic alpha2A-adrenoceptors and dopamine acting at D1 receptors are essential to prefrontal cortex function. Blockade of norepinephrine alpha2-adrenoceptors in prefrontal cortex markedly impairs prefrontal cortex function and mimics most of the symptoms of ADHD, including impulsivity and locomotor hyperactivity. Conversely, stimulation of prefrontal cortical alpha2-adrenoceptors strengthens prefrontal cortex regulation of behavior and reduces distractibility. Thus, effective treatments for ADHD facilitate catecholamine transmission and apparently have their therapeutic actions by optimizing catecholamine actions in the prefrontal cortex"
- ^ Arnsten, A. F. T.; Li, B. M. (2005). "Neurobiology of Executive Functions: Catecholamine Influences on Prefrontal Cortical Functions". Biological Psychiatry 57(11): 1377–1384. doi:10.1016/j.biopsych.2004.08.019. PMID 15950011.
- ^ a b Markowitz JS, Logan BK, Diamond F, Patrick KS (1999). "Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion".Journal of Clinical Psychopharmacology 19 (4): 362–6. doi:10.1097/00004714-199908000-00013. PMID 10440465.
- ^ Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE (2006). "A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder" (PDF).Can J Clin Pharmacol 13 (1): e50–62. PMID 16456216.
- ^ Gilmore A, Milne R (2001). "Methylphenidate in children with hyperactivity: review and cost-utility analysis". Pharmacoepidemiol Drug Saf 10 (2): 85–94.doi:10.1002/pds.564. PMID 11499858.
- ^ Mott TF, Leach L, Johnson L (2004). "Clinical inquiries. Is methylphenidate useful for treating adolescents with ADHD?". The Journal of Family Practice 53 (8): 659–61.PMID 15298843.
- ^ Vitiello B (2001). "Psychopharmacology for young children: clinical needs and research opportunities". Pediatrics 108 (4): 983–9. doi:10.1542/peds.108.4.983.PMID 11581454.
- ^ Hermens DF, Rowe DL, Gordon E, Williams LM (2006). "Integrative neuroscience approach to predict ADHD stimulant response". Expert Review of Neurotherapeutics 6(5): 753–63. doi:10.1586/14737175.6.5.753. PMID 16734523.
- ^ Fone KC, Nutt DJ (2005). "Stimulants: use and abuse in the treatment of ADD". Current Opinion in Pharmacology 5 (1): 87–93. doi:10.1016/j.coph.2004.10.001.PMID 15661631.
- ^ Capp PK, Pearl PL, Conlon C (2005). "Methylphenidate HCl: therapy for attention deficit hyperactivity disorder". Expert Rev Neurother 5 (3): 325–31.doi:10.1586/14737175.5.3.325. PMID 15938665.
- ^ Greenfield B, Hechman L (2005). "Treatment of attention deficit hyperactivity disorder in adults". Expert Rev Neurother 5 (1): 107–21. doi:10.1586/14737175.5.1.107.PMID 15853481.
- ^ Faraone SV, Wilens TE (2007). "Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion". J Clin Psychiatry. 68 Suppl 11: 15–22.PMID 18307377.
- ^ Wilens TE, Faraone SV, Biederman J, Gunawardene S (January 2003). "Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature". Pediatrics 111 (1): 179–85.doi:10.1542/peds.111.1.179. PMID 12509574.
- ^ Schachter HM, Pham B, King J, Langford S, Moher D (2001). "How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis". CMAJ 165 (11): 1475–88. PMC 81663.PMID 11762571.
- ^ Advokat C (July 2010). "What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD)". Neurosci Biobehav Rev 34 (8): 1256–66. doi:10.1016/j.neubiorev.2010.03.006.PMID 20381522.
- ^ a b c Kidd PM (2000). "Attention deficit/hyperactivity disorder (ADHD) in children: rationale for its integrative management" (PDF). Altern Med Rev 5 (5): 402–28.PMID 11056411.
- ^ Stevenson RD, Wolraich ML (1989). "Stimulant medication therapy in the treatment of children with attention deficit hyperactivity disorder". Pediatr. Clin. North Am. 36 (5): 1183–97. PMID 2677938.
- ^ Volkow ND, Wang GJ, Fowler JS, et al. (1998). "Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate". The American Journal of Psychiatry 155 (10): 1325–31. PMID 9766762.
- ^ a b Viggiano D, Vallone D, Sadile A (2004). "Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling". Neural Plasticity 11 (1–2): 102, 106–107.doi:10.1155/NP.2004.97. PMC 2565441. PMID 15303308.Full-text [1]
- ^ Gainetdinov RR, Caron MG (2001). "Genetics of childhood disorders: XXIV. ADHD, part 8: hyperdopaminergic mice as an animal model of ADHD". Journal of the American Academy of Child and Adolescent Psychiatry 40 (3): 380–2. doi:10.1097/00004583-200103000-00020. PMID 11288782.
- ^ Koelega HS (1993). "Stimulant drugs and vigilance performance: a review".Psychopharmacology (Berl.) 111 (1): 1–16. doi:10.1007/BF02257400.PMID 7870923.
- ^ Fry JM (1998). "Treatment modalities for narcolepsy". Neurology 50 (2 Suppl 1): S43–8. PMID 9484423.
- ^ Mitler MM (1994). "Evaluation of treatment with stimulants in narcolepsy". Sleep 17 (8 Suppl): S103–6. PMID 7701190.
- ^ P. Lichtenstein et al. (2012). "Medication for Attention Deficit–Hyperactivity Disorder and Criminality." N Engl J Med 367:2006-2014. DOI: 10.1056/NEJMoa1203241
- ^ E. Pappadopulos et al. (2006)."Pharmacotherapy of aggression in children and adolescents: efficacy and effect size." J Can Acad Child Adolesc Psychiatry. 15(1):27-39.PMID 18392193
- ^ Kraus MF, Burch EA (1992). "Methylphenidate hydrochloride as an antidepressant: controversy, case studies, and review". South. Med. J. 85 (10): 985–91.doi:10.1097/00007611-199210000-00012. PMID 1411740.
- ^ Rozans M, Dreisbach A, Lertora JJ, Kahn MJ (2002). "Palliative uses of methylphenidate in patients with cancer: a review". J. Clin. Oncol. 20 (1): 335–9.doi:10.1200/JCO.20.1.335. PMID 11773187.
- ^ Leonard BE, McCartan D, White J, King DJ (2004). "Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects". Hum Psychopharmacol 19 (3): 151–80. doi:10.1002/hup.579. PMID 15079851.
- ^ Satel SL, Nelson JC (1989). "Stimulants in the treatment of depression: a critical overview". J Clin Psychiatry 50 (7): 241–9. PMID 2567730.
- ^ [2]
- ^ Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J (2008). "Pharmacotherapy of methamphetamine addiction: an update". Substance Abuse 29 (3): 31–49. doi:10.1080/08897070802218554. PMC 2597382. PMID 19042205.
- ^ Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A (1997). "Replacement medication for cocaine dependence: methylphenidate". J Clin Psychopharmacol 17 (6): 485–8. doi:10.1097/00004714-199712000-00008.PMID 9408812.
- ^ Gorelick DA, Gardner EL, Xi ZX (2004). "Agents in development for the management of cocaine abuse". Drugs 64 (14): 1547–73. doi:10.2165/00003495-200464140-00004.PMID 15233592.
- ^ Karila L, Gorelick D, Weinstein A, et al. (2008). "New treatments for cocaine dependence: a focused review". Int. J. Neuropsychopharmacol. 11 (3): 425–38.doi:10.1017/S1461145707008097. PMID 17927843.
- ^ "NIDA InfoFacts: Understanding Drug Abuse and Addiction". 2008.
- ^ Shearer J (2008). "The principles of agonist pharmacotherapy for psychostimulant dependence". Drug Alcohol Rev 27 (3): 301–8. doi:10.1080/09595230801927372.PMID 18368612.
- ^ Journal of Pharmaceutical Sciences, Volume 97, Issue 5, pages 1993–2007, May 2008
- ^ Kaufman, Marc J.; et al., Cocaine-Induced Cerebral Vasoconstriction Detected in Humans With Magnetic Resonance Angiography
- ^ Russo KE, Hall W, Chi OZ, Sinha AK, Weiss HR (1991). "Effect of amphetamine on cerebral blood flow and capillary perfusion". Brain Res 542 (1): 43–8. doi:10.1016/0006-8993(91)90995-8. PMID 1905179.
- ^ Grund T, Lehmann K, Bock N, Rothenberger A, Teuchert-Noodt G (2006). "Influence of methylphenidate on brain development—an update of recent animal experiments".Behav Brain Funct 2: 2. doi:10.1186/1744-9081-2-2. PMC 1363724.PMID 16403217.
- ^ Sagvolden T, Sergeant JA (1998). "Attention deficit/hyperactivity disorder—from brain dysfunctions to behaviour". Behav. Brain Res. 94 (1): 1–10. doi:10.1016/S0166-4328(97)00164-2. PMID 9708834.
- ^ Gordon N (1999). "Attention deficit hyperactivity disorder: possible causes and treatment". Int. J. Clin. Pract. 53 (7): 524–8. PMID 10692738.
- ^ King S, Griffin S, Hodges Z, et al. (2006). "A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents". Health Technol Assess 10 (23): iii–iv, xiii–146. PMID 16796929.
- ^ Gonzalez de Dios J, Cardó E, Servera M (2006). "[Methylphenidate in the treatment of attention-deficit/hyperactivity disorder: are we achieving an adequate clinical practice?]".Rev Neurol (in Spanish; Castilian) 43 (12): 705–14. PMID 17160919.
- ^ – Ritalin Side Effects. Drugs.com. Retrieved on 2011-10-16.
- ^ Jaanus SD (1992). "Ocular side-effects of selected systemic drugs". Optom Clin 2 (4): 73–96. PMID 1363080.
- ^ Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL (2005). "Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study". Sleep 28 (6): 667–72. PMID 16477952.
- ^ Minutes of the FDA Pediatric Advisory Committee. March 22, 2006.
- ^ "FDA may reject safety warning for ADHD drugs". New Scientist. 18 February 2006
- ^ Minutes of the FDA Pediatric Advisory Committee, March 22, 2006
- ^ Kraemer M, Uekermann J, Wiltfang J, Kis B (July 2010). "Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature". Clin Neuropharmacol 33 (4): 204–6.doi:10.1097/WNF.0b013e3181e29174. PMID 20571380.
- ^ a b c d e Characterization of Methylphenidate Exposures Reported to a Regional Poison Control Center - 2000, White & Yadao: Paediatrics & Adolescent Medicine
- ^ a b c d [www.poison.org/stats/2010%20NPDS%20Annual%20Report.pdf Annual report 2010, American Association of Poison Control Centers Toxic Exposure Surveillance System] - Bronstein et al, Table 22B p.136
- ^ a b c d e f g Scharman EJ, Erdman AR, Cobaugh DJ, et al. (2007). "Methylphenidate poisoning: an evidence-based consensus guideline for out-of-hospital management".Clinical Toxicology 45 (7): 737–52. doi:10.1080/15563650701665175.PMID 18058301. [3]
- ^ a b c Severe toxicity due to injected but not oral or nasal abuse of methylphenidate tablets - Bruggisser et al 2011
- ^ Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F (2000). "Trends in the prescribing of psychotropic medications to preschoolers". JAMA 283 (8): 1025–30.doi:10.1001/jama.283.8.1025. PMID 10697062.
- ^ ADHD & Women's Health – Attention-deficit hyperactivity disorder National Women's Health Report. 2003. Retrieved 2007-11-03. "Although methylphenidate is perhaps one of the best-studied drugs available, with thousands of studies attesting to its long-term that finds the numbers of children taking the drug skyrocketing in recent years."
- ^ Edmund J. S. Sonuga-Barke, Margaret Thompson, Howard Abikoff, Rachel Klein, Laurie Miller Brotman. "Nonpharmacological Interventions for Preschoolers With ADHD: The Case for Specialized Parent Training" (PDF). Infants & Young Children 19 (2): 142–153. Retrieved 2008-12-30. "While most recent studies suggest that methylphenidate is relatively well tolerated by young children, some suggest that side-effects might be more marked in preschoolers than in school-aged children (Firestone, Musten, Pisterman, Mercer, & Bennett, 1998). Furthermore, some researchers have argued that there is the potential for negative long-term effects on the developing brains of young children chronically medicated (Moll, Rothenberger, Ruther, & Huther, 2002)."
- ^ Ashton H, Gallagher P, Moore B (2006). "The adult psychiatrist's dilemma: psychostimulant use in attention deficit/hyperactivity disorder". J. Psychopharmacol. (Oxford) 20 (5): 602–10. doi:10.1177/0269881106061710. PMID 16478756.
- ^ Kociancic T, Reed MD, Findling RL (2004). "Evaluation of risks associated with short- and long-term psychostimulant therapy for treatment of ADHD in children". Expert Opin Drug Saf 3 (2): 93–100. doi:10.1517/eods.3.2.93.27337. PMID 15006715.
- ^ "Ritalin & Ritalin-SR Prescribing Information" (PDF). Novartis. 2007.
- ^ Cherland E, Fitzpatrick R (1999). "Psychotic side effects of psychostimulants: a 5-year review" (PDF). Can J Psychiatry 44 (8): 811–3. PMID 10566114.
- ^ Kimko HC, Cross JT, Abernethy DR (1999). "Pharmacokinetics and clinical effectiveness of methylphenidate". Clin Pharmacokinet 37 (6): 457–70.doi:10.2165/00003088-199937060-00002. PMID 10628897.
- ^ Dafny, N.; Yang, P. (2006). "The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects". Brain Research Bulletin 68 (6): 393–405. doi:10.1016/j.brainresbull.2005.10.005.PMID 16459193.
- ^ Ross RG (2006). "Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder". Am J Psychiatry 163 (7): 1149–52.doi:10.1176/appi.ajp.163.7.1149. PMID 16816217.
- ^ DelBello MP, Soutullo CA, Hendricks W, Niemeier RT, McElroy SL, Strakowski SM (2001). "Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset". Bipolar Disord 3 (2): 53–7. doi:10.1034/j.1399-5618.2001.030201.x.PMID 11333062.
- ^ Soutullo CA, DelBello MP, Ochsner JE, et al. (2002). "Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment". J Affect Disord 70 (3): 323–7. doi:10.1016/S0165-0327(01)00336-6.PMID 12128245.
- ^ Kuczenski R, Segal DS (2005). "Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse". Biol. Psychiatry57 (11): 1391–6. doi:10.1016/j.biopsych.2004.12.036. PMID 15950013.
- ^ a b Soto, Paul L.; Kristin M Wilcox, Yun Zhou, Nancy A Ator, Mark A Riddle, Dean F Wong, Michael R Weed (18). "Long-Term Exposure to Oral Methylphenidate or dl-Amphetamine Mixture in Peri-Adolescent Rhesus Monkeys: Effects on Physiology, Behavior, and Dopamine System Development". Neuropsychopharmacology 37: 2566–2579. doi:10.1038/npp.2012.119. PMID 22805599. Retrieved 2 December 2012.
- ^ Roberts SM, DeMott RP, James RC (1997). "Adrenergic modulation of hepatotoxicity".Drug Metab. Rev. 29 (1–2): 329–53. doi:10.3109/03602539709037587.PMID 9187524.
- ^ Marotta PJ, Roberts EA (1998). "Pemoline hepatotoxicity in children". The Journal of Pediatrics 132 (5): 894–7. doi:10.1016/S0022-3476(98)70329-4. PMID 9602211.
- ^ Patrick KS, González MA, Straughn AB, Markowitz JS (2005). "New methylphenidate formulations for the treatment of attention-deficit/hyperactivity disorder". Expert Opinion on Drug Delivery 2 (1): 121–43. doi:10.1517/17425247.2.1.121. PMID 16296740.
- ^ Markowitz JS, DeVane CL, Boulton DW, et al. (2000). "Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol". Drug Metabolism and Disposition 28 (6): 620–4. PMID 10820132.
- ^ Markowitz, J. S.; Devane, C. L.; Boulton, D. W.; Nahas, Z.; Risch, S. C.; Diamond, F.; Patrick, K. S. (2000). "Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol". Drug metabolism and disposition: the biological fate of chemicals 28 (6): 620–624. PMID 10820132.
- ^ a b Patrick KS, Straughn AB, Perkins JS, González MA (2009). "Evolution of stimulants to treat ADHD: transdermal methylphenidate". Human Psychopharmacology 24 (1): 1–17. doi:10.1002/hup.992. PMC 2629554. PMID 19051222.
- ^ Patrick KS, Williard RL, VanWert AL, Dowd JJ, Oatis JE, Middaugh LD (2005). "Synthesis and pharmacology of ethylphenidate enantiomers: the human transesterification metabolite of methylphenidate and ethanol". Journal of Medicinal Chemistry 48 (8): 2876–81. doi:10.1021/jm0490989. PMID 15828826.
- ^ Williard RL, Middaugh LD, Zhu HJ, Patrick KS (2007). "Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity". Behavioural Pharmacology 18 (1): 39–51.doi:10.1097/FBP.0b013e3280143226. PMID 17218796.
- ^ a b Tan M, Appleton R (2005). "Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy". Archives of Disease in Childhood 90 (1): 57–9.doi:10.1136/adc.2003.048504. PMC 1720074. PMID 15613514.
- ^ Methylphenidate Use During Pregnancy and Breastfeeding. Drugs.com. Retrieved on 2011-04-30.
- ^ Humphreys C, Garcia-Bournissen F, Ito S, Koren G (2007). "Exposure to attention deficit hyperactivity disorder medications during pregnancy". Canadian Family Physician53 (7): 1153–5. PMC 1949295. PMID 17872810.
- ^ Kaufman, David Myland; Heinonen, Olli P.; Slone, Dennis; Shapiro, Samuel (1977).Birth defects and drugs in pregnancy. Littleton, Mass: Publishing Sciences Group.ISBN 0-88416-034-3. OCLC 2387745.[page needed]
- ^ Yaffe, Sumner J.; Briggs, Gerald G.; Freeman, Roger Anthony (2005). Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-5651-0.[page needed]
- ^ a b Klein-Schwartz W (2002). "Abuse and toxicity of methylphenidate". Current Opinion in Pediatrics 14 (2): 219–23. doi:10.1097/00008480-200204000-00013.PMID 11981294.
- ^ https://www.erowid.org/pharms/methylphenidate/methylphenidate_info1.shtml
- ^ a b c 2004 Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System - Table 22B p.652
- ^ Safety of therapeutic methylphenidate in adults: a systematic review of the evidence- 2008, Godfrey
- ^ a b Stern EJ, Frank MS, Schmutz JF, Glenny RW, Schmidt RA, Godwin JD (1994). "Panlobular pulmonary emphysema caused by i.v. injection of methylphenidate (Ritalin): findings on chest radiographs and CT scans". American Journal of Roentgenology 162(3): 555–60. PMID 8109495.
- ^ Leith NJ, Barrett RJ (1981). "Self-stimulation and amphetamine: tolerance to d and l isomers and cross tolerance to cocaine and methylphenidate". Psychopharmacology (Berl.) 74 (1): 23–8. doi:10.1007/BF00431751. PMID 6791199.
- ^ Cohen D, Leo J, Stanton T, et al. (2002). "A boy who stops taking stimulants for "ADHD": commentaries on a Pediatrics case study". Ethical Hum Sci Serv 4 (3): 189–209. PMID 15278983.
- ^ Schwartz RH, Rushton HG (2004). "Stuttering priapism associated with withdrawal from sustained-release methylphenidate". J. Pediatr. 144 (5): 675–6.doi:10.1016/j.jpeds.2003.12.039. PMID 15127013.
- ^ Garland EJ (1998). "Pharmacotherapy of adolescent attention deficit hyperactivity disorder: challenges, choices and caveats". J. Psychopharmacol. (Oxford) 12 (4): 385–95. doi:10.1177/026988119801200410. PMID 10065914.
- ^ Nolan EE, Gadow KD, Sprafkin J (1999). "Stimulant medication withdrawal during long-term therapy in children with comorbid attention-deficit hyperactivity disorder and chronic multiple tic disorder". Pediatrics 103 (4 Pt 1): 730–7.doi:10.1542/peds.103.4.730. PMID 10103294.
- ^ Smucker WD, Hedayat M (2001). "Evaluation and treatment of ADHD". Am Fam Physician 64 (5): 817–29. PMID 11563573.
- ^ Rosenfeld AA (1979). "Depression and psychotic regression following prolonged methylphenidate use and withdrawal: case report". Am J Psychiatry 136 (2): 226–8.PMID 760559.
- ^ Riccio CA, Waldrop JJ, Reynolds CR, Lowe P (2001). "Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation". J Neuropsychiatry Clin Neurosci 13 (3): 326–35.doi:10.1176/appi.neuropsych.13.3.326. PMID 11514638.
- ^ Yang. "Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization". Retrieved 2 April 2013.
- ^ Featherstone, R. E.; Kapur, S.; Fletcher, P. J. (2007). "The amphetamine-induced sensitized state as a model of schizophrenia". Progress in Neuro-Psychopharmacology and Biological Psychiatry 31 (8): 1556–1571. doi:10.1016/j.pnpbp.2007.08.025.PMID 17884274.
- ^ a b Volkow, N. D.; Wang, G. J.; Fowler, J. S.; Gatley, S. J.; Logan, J.; Ding, Y. S.; Dewey, S. L.; Hitzemann, R.; Gifford, A. N.; Pappas, N. R. (1999). "Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of "high"". The Journal of Pharmacology and Experimental Therapeutics 288 (1): 14–20.PMID 9862747.
- ^ Volkow ND, Swanson JM (2003). "Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD". The American Journal of Psychiatry 160(11): 1909–18. doi:10.1176/appi.ajp.160.11.1909. PMID 14594733.
- ^ Morton WA, Stockton GG (2000). "Methylphenidate Abuse and Psychiatric Side Effects". Primary Care Companion Journal of Clinical Psychiatry 2 (5): 159–64.
- ^ Arria AM, Wish ED (2006). "Nonmedical use of prescription stimulants among students". Pediatric Annals 35 (8): 565–71. PMC 3168781. PMID 16986451.
- ^ Midgely, Carol (February 21, 2003). "Kiddie coke: A new peril in the playground".The Times (London). Retrieved 21 February 2010.[unreliable medical source?]
- ^ Harris J (2009). "Is it acceptable for people to take methylphenidate to enhance performance? Yes". BMJ 338: b1955. doi:10.1136/bmj.b1955. PMID 19541705.
- ^ Chatterjee A (2009). "Is it acceptable for people to take methylphenidate to enhance performance? No". BMJ 338: b1956. doi:10.1136/bmj.b1956. PMID 19541706.
- ^ "Ritalin backed as brain-booster". BBC News. 19 June 2009. Retrieved 21 February 2010.
- ^ Davies, Caroline (21 February 2010). "Universities told to consider dope tests as student use of 'smart drugs' soars". The Observer (London). Retrieved 21 February 2010.
- ^ Green List: Annex to the annual statistical report on psychotropic substances (form P) PDF (1.63 MB) 23rd edition. August 2003. International Narcotics Board, Vienna International Centre. Retrieved 2 March 2006.
- ^ "Misuse of Drugs Act 1971 (c. 38): SCHEDULE 2: Controlled Drugs". Office of Public Sector Information. Retrieved 2009-06-15.
- ^ a b Moses, Scott (2009-07-26). "Methylphenidate". Family Practice Notebook. Retrieved 2012-08-07. "Duration: 5-8 hours (gradual decrease after 3 hours)".
- ^ "Education/Training » Clinical Resources". Illinois DocAssist website. University of Illinois at Chicago. Retrieved 2012-07-26. "Ritalin‑SR, methylphenidate SR, Methylin ER, and Metadate ER are the same formulation and have the same drug delivery system".
- ^ "Apo‑Methylphenidate SR product monograph" (PDF). Apotex Inc. 2005-03-31. "Comparative Bioavailability" section. Retrieved 2012-07-26. If the monograph link doesn't work, visit Health Canada's Drug Product Database query form one time, then click the monograph link again.
- ^ "New product: Sandoz Methylphenidate SR 20 mg". Sandoz Canada Inc. 2009-05-05. Retrieved 2012-07-26. "An alternative to Ritalin‑SR from Novartis".
- ^ Concerta for Kids with ADHD. Pediatrics.about.com (2003-04-01). Retrieved on 2011-04-30.
- ^ Concerta (Methylphenidate Extended-Release Tablets) Drug Information: User Reviews, Side Effects, Drug Interactions and Dosage at RxList. Rxlist.com. Retrieved on 2011-04-30.
- ^ Ritalin LA® (methylphenidate hydrochloride) extended-release capsules, Novartis
- ^ Metadate CD. Adhd.emedtv.com. Retrieved on 2011-04-30.
- ^ Lakhan SE, Hagger-Johnson GE (2007). "The impact of prescribed psychotropics on youth". Clin Pract Epidemol Ment Health 3 (1): 21. doi:10.1186/1745-0179-3-21.PMC 2100041. PMID 17949504.
- ^ New Research Helps Explain Ritalin's Low Abuse Potential When Taken As Prescribed – 09/29/1998. Nih.gov. Retrieved on 2011-04-30.
- ^ Stimulant ADHD Medications: Methylphenidate and Amphetamines – InfoFacts – NIDA. Drugabuse.gov. Retrieved on 2011-04-30.
- ^ Weinberg WA, Brumback RA (1992). "The myth of attention deficit-hyperactivity disorder: symptoms resulting from multiple causes". J. Child Neurol. 7 (4): 431–45; discussion 446–61. doi:10.1177/088307389200700420. PMID 1469255.
- ^ Ouellette EM (1991). "Legal issues in the treatment of children with attention deficit hyperactivity disorder". Journal of Child Neurology. 6 Suppl: S68–75. PMID 2002217.
- ^ FDA OKs Concerta for Adult ADHD, webmd.com
- ^ Ritalin for Adults. Adhd.emedtv.com (2007-03-06). Retrieved on 2011-04-30.
- ^ Drug Enforcement Administration, Greene, S.H., Response to CHADD petition concerning Ritalin, 1995, August 7. Washington, DC: DEA, U.S. Department of Justice.
- ^ The Neurobiology of ADHD, ADHD.org.nz
- ^ GARDINER HARRIS (2012-02-03). "F.D.A. Finds Short Supply of Attention Deficit Drugs". The New York Times. Archived from the original on 2012-02-03. Retrieved 2012-02-03. "(Archived by WebCite® at [4])"
- ^ L. ALAN SROUFE (2012-01-28). "Ritalin Gone Wrong - Opinion - Children\'s A.D.D.Drugs Don\'t Work Long-Term". The New York Times. Archived from the original on 2012-02-03. Retrieved 2012-02-03. "(Archived by WebCite® at [5])"
- ^ Presenters: Joan Hamburg and Dr. Harold Koplewicz (2012-02-03). "Are we over medicating our kids? Speaking with Dr. Harold Koplewicz of @ChildMindDotOrg". Joan Hamburg Show (Radio). 1:11 into the complete two part show - 2 minutes in. WOR Radio Network. WOR.
- ^ a b c Heal DJ, Pierce DM (2006). "Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system".CNS Drugs 20 (9): 713–38. doi:10.2165/00023210-200620090-00002.PMID 16953648.
- ^ Froimowitz M, Patrick KS, Cody V (1995). "Conformational analysis of methylphenidate and its structural relationship to other dopamine reuptake blockers such as CFT".Pharmaceutical Research 12 (10): 1430–4. doi:10.1023/A:1016262815984.PMID 8584475.
- ^ Prashad, M (2001). "Approaches to the Preparation of Enantiomerically Pure (2R,2′R)-(+)-threo-Methylphenidate Hydrochloride". Adv. Synth. Catal 343 (5): 379–92.doi:10.1002/1615-4169(200107)343:5<379::AID-ADSC379>3.0.CO;2-4.
- ^ Axten, J. M.; Krim, L.; Kung, H. F.; Winkler, J. D. (1998). "A Stereoselective Synthesis of dl-threo-Methylphenidate: Preparation and Biological Evaluation of Novel Analogues".The Journal of Organic Chemistry 63 (26): 9628. doi:10.1021/jo982214t.
- ^ Singh, Satendra (2000). "Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists". Chem. Rev. 100 (3): 925–1024 (1008).doi:10.1021/cr9700538. PMID 11749256.
- ^ Panizzon, Leandro (1944). "La preparazione di piridil- e piperidil-arilacetonitrili e di alcuni prodotti di trasformazione (Parte Ia)". Helvetica Chimica Acta 27: 1748–56.doi:10.1002/hlca.194402701222.
- ^ Meier, R; Gross, F; Tripod, J (1954). "Ritalin, a new synthetic compound with specific analeptic components". Klinische Wochenschrift 32 (19–20): 445–50.PMID 13164273.
- ^ Myers, Richard L (2007-08). The 100 most important chemical compounds: a reference guide By Richard L. Myers. ISBN 978-0-313-33758-1. Retrieved 2010-09-10.
- ^ Leandro Panizzon et al Pyridine and piperdjine compounds U.S. Patent 2,507,631Issue date: May 16, 1950
- ^ Rudolf Rouietscji et al Process for the conversion of U.S. Patent 2,838,519 Issue date: Jun 10, 1958
- ^ Rudolf Rouietscji et alProcess for the conversion of U.S. Patent 2,957,880 Issue date: Oct 25, 1960
- ^ Terrance Woodworth (May 16, 2000). "DEA Congressional Testimony". Retrieved 2007-11-02.
- ^ Approved Drug Therapies (637) Concerta, Alza. CenterWatch. Retrieved on 2011-04-30.
- ^ Iversen L (2006). "Neurotransmitter transporters and their impact on the development of psychopharmacology". British Journal of Pharmacology 147 (Suppl 1): S82–8.doi:10.1038/sj.bjp.0706428. PMC 1760736. PMID 16402124.
- ^ Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x.PMC 3005101. PMID 21073468.
- ^ Novartis:Focalin XR Overview
- ^ Focalin XR – Full Prescribing Information. Novartis.
- ^ Concerta XL 18 mg – 36 mg prolonged release tablets last updated on the eMC: 05/11/2010
- ^ Sulzer D, Sonders MS, Poulsen NW, Galli A (2005). "Mechanisms of neurotransmitter release by amphetamines: a review" (PDF). Prog. Neurobiol. 75 (6): 406–33.doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
- ^ Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R (2006). "A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study". J Child Adolesc Psychopharmacol 16 (6): 687–98.doi:10.1089/cap.2006.16.687. PMID 17201613.
- ^ T. J. Volz (2008). "Neuropharmacological Mechanisms Underlying the Neuroprotective Effects of Methylphenidate". Current Neuropharmacology.doi:10.2174/157015908787386041. PMC 2701286.
- ^ Rosack Jim (2 January 2004). "Brain Scans Reveal Physiology of ADHD".Psychiatric News 39 (1): 26.
- ^ Liddle, E. B.; Hollis, C.; Batty, M. J.; Groom, M. J.; Totman, J. J.; Liotti, M.; Scerif, G.; Liddle, P. F. (2011). "Task-related default mode network modulation and inhibitory control in ADHD: Effects of motivation and methylphenidate". Journal of Child Psychology and Psychiatry 52 (7): 761–771. doi:10.1111/j.1469-7610.2010.02333.x.PMID 21073458.
- ^ Kimko, H. C.; Cross, J. T.; Abernethy, D. R. (1999). "Pharmacokinetics and Clinical Effectiveness of Methylphenidate". Clinical Pharmacokinetics 37 (6): 457.doi:10.2165/00003088-199937060-00002. PMID 10628897.
- ^ Chan, Y. P.; Swanson, J. M.; Soldin, S. S.; Thiessen, J. J.; MacLeod, S. M.; Logan, W. (1983). "Methylphenidate hydrochloride given with or before breakfast: II. Effects on plasma concentration of methylphenidate and ritalinic acid". Pediatrics 72 (1): 56–59.PMID 6866592.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, pp. 1091–93.






I want to share with you all on how Dr Itua saves my life with his powerful Herbal medicines, I was diagnosed of Oral/Ovarian Cancer which i suffered from for 5 years with no positive treatment until when My son came to me in the hospital when i was laying down on my dying bed waiting for god to call out my name to join him in heaven.
ОтветитьУдалитьMy son was so excited that very day he came across Dr Itua on Blogspot, we decided to give him a try although we Americans are so scared to trust Africans but i really have no choice that time to choose life in between so we gave a try to Dr Itua Herbal medicines, god wiling he was a good man with a god gift. Dr Itua send us the herbal medicine it was three bottles. I take it for three weeks instructor and this herbal medicines heal me, cure my Oral/Ovarian Cancer completely I have been living for 9 months now with healthy life no more symptoms.
I'm sponsoring Dr Itua in LA Advert on Cancer patent seminar which my son will be participating too and other patent Dr Itua has cured from all kind of human disease, also if you are sick from disease like,Epilepsy,Breast Cancer,Prostate Cancer,Throat cancer,Thyroid Cancer,Uterine cancer,Fibroid,Angiopathy, Ataxia,Arthritis,Brain cancer,Hiv,. Vaginal cancer,Herpes,Colon-Rectal Cancer,Chronic Disease.Amyotrophic Lateral Scoliosis,Brain Tumor,Fibromyalgia,Fluoroquinolone Toxicity,Multiple myeloma,Tach Diseases,Leukemia,Liver cancer,
Esophageal cancer,Gallbladder cancer,,Bladder cancer,Gestational trophoblastic disease,Head and neck cancer,Hodgkin lymphoma
Intestinal cancer,Kidney cancer,Hpv,Lung cancer,Adrenal cancer.Bile duct cancer,Bone cancer,Melanoma,Mesothelioma,Neuroendocrine tumors
Non-Hodgkin lymphoma,Cervical Cancer,Oral cancer,Hepatitis,Skin cancer,Soft tissue sarcoma,Spinal cancer,Pancreatic Cancer, Stomach cancer
Testicular cancer,
Syndrome Fibrodysplasia Ossificans ProgresSclerosis,Alzheimer's disease,Chronic Diarrhea,Copd,Parkinson,Als,Adrenocortical carcinoma Infectious mononucleosis,Vulvar cancer,Ovarian cancer,,Sinus cancer, Here Is The Wonderful Healer Contact. Name_ Doctor Itua, Email Contact: drituaherbalcenter@gmail.com, Phone/WhatsApp: +2348149277967